What is in this leaflet
This leaflet answers some common questions about SEPTRIN. It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
Taking any medicine involves some risk. You doctor has weighted the risks of you using SEPTRIN against the benefits they expect it will have for you.
If you have any concerns about taking SEPTRIN, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may want to read it again.
What SEPTRIN is used for
SEPTRIN is used for the treatment of a variety of bacterial infections, including bronchitis and infections of the ear, sinus, kidney, bladder, stomach, bowel, skin and wounds.
Trimethoprim and sulfamethoxazole (the active ingredients in SEPTRIN) belong to a group of medicines called "anti-infectives". They work in slightly different ways to stop the growth of bacteria causing the infection.
If you have any questions about why you are taking SEPTRIN, ask your doctor or pharmacist. Your doctor may have prescribed it for another reason.
There is no evidence that this medicine is addictive.
SEPTRIN is only available with a doctor’s prescription.
Before taking it
When you must not take it
Do not use SEPTRIN if you have an allergy to:
- trimethoprim
- Sulfamethoxazole
- any other sulfonamide (sulfur) antibiotic
- Any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include: shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue which may cause difficulty in swallowing or breathing; rash, itching or hives, peeling of the skin.
Do not use SEPTRIN if:
You have severe liver or kidney disease, any blood disorder or megaloblastic anaemia.
The child you are treating is under 3 months of age.
You have streptococcal Pharyngitis.
You are taking dofetilide, a medicine used to treat irregular heartbeats.
Do not take SEPTRIN after the expiry date printed on the pack or bottle. SEPTRIN may have no effect if you take it after the expiry date.
Do not use SEPTRIN if the packaging appears to have been tampered with.
Do not use SEPTRIN to treat any other conditions unless advised by your doctor.
Do not give SEPTRIN to anyone else, even if their symptoms seem similar to yours.
Before you start to use it
You must tell your doctor if:
You are allergic to:
- trimethoprim, sulfamethoxazole or any other ingredient listed at the end of this information.
- any other sulphonamide type antibiotics, any diuretics (medicines that increase the volume of your urine), or medicines for diabetes or an overactive thyroid gland.
You are pregnant or breastfeeding or become pregnant, while taking SEPTRIN.
You have ever had any type of liver, kidney or bladder complaint or disease (eg. hepatitis).
You have ever had any type of blood disorder (including porphyria and glucose-6-phosphate dehydrogenase deficiency).
You have asthma or an allergic disorder.
You have a folic acid vitamin deficiency.
You have phenylketonuria.
You suffer from alcoholism.
You suffer from rheumatoid arthritis.
You are being treated for epilepsy (fits).
You have been diagnosed with AIDS. People with AIDS may not tolerate or respond to SEPTRIN in the same manner as someone who does not have AIDS. There may be an increase in the number and severity of side effects.
You are taking any medicines, including:
- Any malaria medicines (eg. pyrimethamine)
- Any diabetes medicines such as repaglinide, rosiglitazone, pioglitazone, glibenclamide, gliclazide, glipizide, chlorpropamide and tolbutamide
- Any diuretics (diuretics)
- Any epilepsy (fits) medicines (eg. phenytoin, primidone and some barbiturates)
- Any medicines used to treat infections such as rifampicin, dapsone and polymyxin
- Zidovudine, a medicine to treat HIV infection
- Ciclosporin, a medicine used to prevent organ transplant rejections or to treat certain problems with the immune system
- Warfarin, acenocoumarol, phenprocoumon, medicines used to thin the blood
- Medicines used to treat some heart conditions such as digoxin and amiodarone
- Amantadine, a medicine commonly used to treat the influenza virus and Parkinson's disease
- Memantine, a medicine used to treat Parkinson's disease
- Lamivudine, an antiretroviral medicine used to treat HIV/AIDS
- Urinary acidifiers (for kidney conditions) oral contraceptives (‘the pill’)
- Sulfinpyrazone, a medicine used to treat gout
- Salicylates, medicines to treat conditions such as psoriasis or warts
- Medicines used to treat cancer such as paclitaxel, mercaptopurine and methotrexate
- Clozapine, a medicine used to treat schizophrenia
- Medicines used to treat overactive thyroid conditions
- Medicines used to treat depression such as imipramine, clomipramine, amitriptyline, dosulepin (dothiepin), doxepin, nortriptyline and trimipramine
- Immunosuppressant medicines such as azathioprine and methotrexate
- Medicines used to treat high blood pressure as well as a variety of heart and kidneyconditions such as captopril, enalapril, lisinopril, fosinopril, perindopril, quinapril, ramipril, trandolapril, valsartan, telmisartan, irbesartan, candesartan, eprosartan, losartan, dofetilide and olmesartan
- Procainamide
These medicines may be affected by SEPTRIN or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines. Your doctor will advise you.
You are taking any other medicines, including medicines that you buy without a prescription from a pharmacy, supermarket or health food shop.
Your doctor will have a complete list of the medicines that may cause problems when taken with SEPTRIN.
SEPTRIN and very young children
SEPTRIN should not be used in premature babies nor during the first six weeks of life. It should probably not be given into infants less than 3 months of age.
SEPTRIN and the elderly
The use of SEPTRIN in elderly patients, particularly those with a liver or kidney disease or those taking certain other medicines such as diuretics, carries an increased risk of severe adverse reactions.
How to take it
How much to take
Take SEPTRIN as directed by your doctor or pharmacist.
For adults and children over 12 years old the usual dose is one SEPTRIN Forte Tablet, twice a day.
Depending on the type of infection, and your overall health, your doctor may prescribe a different dose of SEPTRIN. Always take the full course of SEPTRIN prescribed by your doctor.
How to take it
SEPTRIN Forte Tablets should be taken with a glass of water.
Do not crush or chew the tablets.
If you forget to take it
If it is almost time for your next dose, skip the dose that you have missed and take your next dose when you are supposed to.
Otherwise take the dose that you have missed as soon as you remember, then go back to your normal schedule.
If you have forgotten to take more than one dose, contact your doctor or pharmacist.
Do not take a double dose to make up for the dose that you have missed.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately contact your doctor or Poisons Information Centre for advice, or go to Casualty at your nearest hospital, if you think that you or anyone else may have taken too much SEPTRIN. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep telephone numbers for these places handy.
If too much SEPTRIN has been taken signs and symptoms may include nausea, vomiting, dizziness, headache, depression and confusion. It is also possible that you will feel drowsy and you may lose consciousness.
While you are taking it
Things you must do
It is important that you drink plenty of fluids (water) while you are taking SEPTRIN.
Tell your doctor that you are taking SEPTRIN before you have any blood tests. If you are taking SEPTRIN over a long period, or you have kidney problems, your doctor may ask you to undergo regular blood and urine tests.
Your doctor or pharmacist will be able to tell you whether there are any other special instructions while you are taking SEPTRIN.
If you become pregnant while taking SEPTRIN, tell your doctor.
Tell your doctor if you fell the tablets is not helping your condition.
Tell your doctor or pharmacist immediately if you get severe diarrhoea, even if it occurs several weeks after stopping SEPTRIN.
Do not take any diarrhoea medicine without checking with your doctor. Diarrhoea may mean that you have a serious condition affecting your bowel. You may need urgent medical care.
Things you must not do
Do not stop taking SEPTRIN just because you feel better.
It is important that you take the full course of SEPTRIN prescribed by your doctor. This will reduce the risk of your infection recurring.
Your doctor will advise you when to stop taking SEPTRIN.
If you are unsure whether you should stop taking SEPTRIN, talk to your doctor or pharmacist.
Things to be careful of
Be careful driving or operating machinery until you know how SEPTRIN affects you.
Sometimes use of this medicine allows other bacteria and fungi which are not sensitive to SEPTRIN grow. If other infections such as thrush occur while you are taking SEPTRIN tell your doctor.
Be careful drinking alcohol while taking SEPTRIN. Combining SEPTRIN and alcohol can make you feel sick, vomit, or have stomach cramps, headaches and flushing. Your doctor may suggest you avoid alcohol while being treated with SEPTRIN.
Your skin may burn more easily while you are taking SEPTRIN. If outdoors, wear protective clothing or use a SPF 30+ sunscreen.
Side Effects
Stop taking SEPTRIN and tell your doctor immediately if you notice, or experience, any of the following (you may need urgent medical attention):
- Any form of skin rash.
The rash may indicate that you are allergic to SEPTRIN. In very rare circumstances, patients have died from complications that may arise from certain severe allergic reactions. Your doctor will know what action to take if you develop a severe reaction. - Severe fever, chills, sore throat, joint pains, cough or bruising.
- Difficulty breathing or chest pains.
- Severe, persistent diarrhoea. This is particularly important if there is any blood or mucus present. It is possible for this reaction to only become apparent several weeks after taking SEPTRIN.
- Jaundice (yellowing of the skin), which is related to the function of your liver.
- Severe persistent headache
- Discolouration of urine
SEPTRIN may also cause more common side effects such as nausea, vomiting, diarrhoea or other abdominal (gut) or stomach discomfort and inflammation of the mouth or tongue.
These side effects are not usually serious or long lasting.
Tell your doctor if you notice these side effects and they worry you:
- oral thrush (white, furry sore tongue and mouth)
- vaginal thrush (sore and itchy vagina, vaginal discharge).
Your doctor will need to treat the thrush infection separately.
Other rare side effects include various other allergic side effects, pins and needles in the hands and feet, ringing in the ears, diarrhoea, loss of appetite, kidney damage, fits, headaches, depression, imagined sensations (hallucinations), nervousness, an increase or decrease in urine production, unsteadiness, dizziness, sleeplessness, weakness, tiredness, increased sensitivity to light, respiratory toxicity (Acute Respiratory Distress Syndrome) and stomach pains.
If you experience any of these side effects, please contact your doctor as soon as possible.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Tell your doctor if you notice anything else that is making you feel unwell, even if it is not on this list.
Ask your doctor or pharmacist if you don't understand anything in this list.
Various effects on the blood, particularly in the elderly, have been attributed to the use of SEPTRIN. In very rare circumstances, patients have died from some of these effects.
Having a blood test may be able to detect these effects.
Tell your doctor or pharmacist as soon as possible if you think you are experiencing any side effects from taking SEPTRIN.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
After using it
If your doctor advises you to stop taking SEPTRIN, ask your pharmacist what to do with any SEPTRIN that you may have left.
Storage
Keep your tablets in a cool dry place where the temperature stays below 30°C. Protect the tablets from light.
Keep your SEPTRIN in the container it was supplied with.
Do not store SEPTRIN in the bathroom or near a sink. Heat and dampness may affect this medicine.
Do not leave SEPTRIN in the car on hot days.
Keep SEPTRIN and all other medicines, where children cannot reach them.
Product description
What it looks like
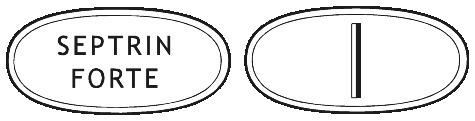
SEPTRIN Forte Tablets are white, elliptical, biconvex, and embossed “SEPTRIN FORTE” on the upper face and the bottom face is plain and scored along the shorter axis.
They are supplied in blister packs of 10 tablets.
Ingredients
Each SEPTRIN Forte Tablet contains 160 mg of trimethoprim and 800 mg sulfamethoxazole.
The tablets also contain:
- povidone
- docusate sodium
- sodium starch glycollate
- magnesium stearate
The Australian Registration numbers are:
Septrin Forte Tablets: AUST R 10998
Supplier
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
Australia
www.arrotex.com.au
This leaflet was revised in May 2023.
Published by MIMS July 2023





 Chemical Name: 2,4-diamino-5-(3,4,5,trimethoxybenzyl)-pyrimidine.
Chemical Name: 2,4-diamino-5-(3,4,5,trimethoxybenzyl)-pyrimidine. Chemical Name: N1-(5-methyl-3-isoxazolyl) sulphanilamide.
Chemical Name: N1-(5-methyl-3-isoxazolyl) sulphanilamide.