What is in this leaflet
This leaflet answers some common questions about this medicine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
Sevelamer is used to treat hyperphosphatemia, a condition caused by too much dietary phosphorus being retained in your body due to a diseased kidney.
Increased levels of serum phosphorus can lead to hard deposits in your body called calcification. These deposits can stiffen your blood vessels and make it harder for blood to be pumped around the body.
Sevelamer helps to remove excess phosphorus that has built up in your body by binding to the phosphorus that is in the food that you eat.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor's prescription.
Before you take this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- any medicine containing any sevelamer carbonate
- any other similar medicine, such as sevelamer hydrochloride
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine if you are pregnant or breastfeeding until you have spoken to your doctor. Your doctor can discuss with you the risks and benefits involved.
Do not take this medication if you have any of the following medication conditions:
- hypophosphatemia, a condition where you do not have enough phosphorus in your body
- bowel obstruction
Do not give this medicine to a child under the age of 18 years. Safety and effectiveness in children younger than 18 years have not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- swallowing problems
- severe constipation
- problems with movement in your stomach and bowel
- active inflammation of the bowel
- undergone major surgery on your stomach or bowel
- thyroid problems
- you have or have had any other medical conditions
Tell your doctor if you are pregnant or plan to become pregnant or are breastfeeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and this one may interfere with each other. These include:
- immunosuppressants, such as cyclosporin, mycophenolate mofetil and tacrolimus – the effects of these medicines may be reduced by sevelamer
- ciprofloxacin, an antibiotic used to treat some bacterial infections
- medications for thyroid, such as levothyroxine – your doctor may need to monitor the levels of thyroid stimulating hormone (TSH) in your blood more closely
- medications for heart rhythm problems
- medications for epilepsy
These medicines may be affected by sevelamer or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take this medicine
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the directions, ask your doctor or pharmacist for help.
How much to take
The recommended starting dose of sevelamer is 2.4 g to 4.8 g per day to be taken over 3 meals.
For tablets, this means one to two 800 mg tablets with each meal three times a day.
The dose will depend on your serum phosphorus level.
How to take it
Swallow the tablets whole with a full glass of water.
Do not crush, chew or break into pieces.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you remember when to take it.
It is best to take sevelamer with meals.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
Sevelamer helps lower your dietary phosphate but does not cure your condition. Therefore, you must continue to take your medicine as directed by your doctor if you expect to lower your phosphate level and keep it down.
If you stop taking sevelamer, your phosphate levels may rise again. It is important to keep taking your medicines even if you feel well.
If you forget to take it
If you miss a dose, take the next dose at the usual time with your meal.
Do not take a double dose to make up for the dose that you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much of this medicine. Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
While you are using this medicine
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking this medicine.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant or start to breastfeed while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all your doctor's appointments so that your progress can be checked. Your doctor will check your progress and monitor your phosphorus levels from time to time to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not take this medicine to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor. Your doctor may need to change the dose of your other medicines if you stop taking sevelamer, so you should only stop when your treating doctor tells you to.
Things to be careful of
Due to either your kidney condition or your dialysis treatment you may:
- develop low or high levels of calcium in your blood – since this medicine does not contain calcium your doctor may prescribe additional calcium tablets
- have a low amount of vitamin D in your blood – your doctor may monitor the levels of vitamin D in your blood and prescribe additional vitamin D as necessary. If you do not take multivitamin supplements you may also develop low levels of vitamins A, E, K and folic acid in your blood and therefore your doctor may monitor these levels and prescribe supplemental vitamins as necessary
- develop peritonitis (infection of your abdominal fluid) associated with your peritoneal dialysis – this risk can be reduced by careful adherence to sterile techniques during bag changes. You should tell your doctor immediately if you experience any new signs or symptoms of abdominal distress, abdominal swelling, abdominal tenderness, constipation, fever, chills, nausea or vomiting.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
If you are over 65 years of age you may have an increased chance of getting side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- vomiting
- nausea
- constipation
- diarrhoea
- flatulence
- indigestion
- abdominal pain
The above list includes the more common side effects of your medicine.
Tell your doctor as soon as possible if you notice any of the following:
- rash, itching or hives on the skin
- severe constipation
The above list includes serious side effects that may require medical attention.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- symptoms of an allergic reaction including cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Some of these side effects can only be found when your doctor does tests from time to time to check your progress.
Storage and Disposal
Storage
Keep your tablets in their original packaging until it is time to take them. If you take the tablets out of their original packaging they may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 25°C.
Do not store this medicine or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
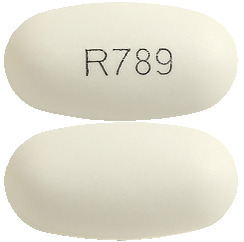
What ARX-Sevelamer looks like
ARX-Sevelamer tablets are white to off-white oval film- coated tablets imprinted with 'R789' on one side and are plain on the other side. AUST R 286648.
Ingredients
This medicine contains 800 mg of sevelamer carbonate as the active ingredient.
This medicine also contains the following:
- mannitol
- crospovidone
- zinc stearate
- hyprolose
- silicon dioxide
- polyvinyl alcohol
- purified talc
- lecithin
- xanthan gum
- purified water
This medicine does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Supplier
Arrotex Pharmaceuticals Pty Ltd
15 - 17 Chapel Street
Cremorne VIC 3121
Web: http://arrotex.com.au
This leaflet was last updated in October 2023.
Published by MIMS January 2024

 For patients previously on calcium based phosphate binders, Table 2 provides guidance on switching to sevelamer carbonate. Serum phosphorus levels should be monitored to ensure optimal daily doses.
For patients previously on calcium based phosphate binders, Table 2 provides guidance on switching to sevelamer carbonate. Serum phosphorus levels should be monitored to ensure optimal daily doses.
 In clinical practice, treatment will be continuous based on the need to control serum phosphorus levels and the daily dose is expected to be an average of approximately 6 g per day.
In clinical practice, treatment will be continuous based on the need to control serum phosphorus levels and the daily dose is expected to be an average of approximately 6 g per day. The most frequently occurring (≥ 2% of patients) undesirable effects possibly or probably related to sevelamer were mainly in the gastrointestinal disorders system organ class (Table 5). Most of these adverse reactions were mild to moderate in intensity.
The most frequently occurring (≥ 2% of patients) undesirable effects possibly or probably related to sevelamer were mainly in the gastrointestinal disorders system organ class (Table 5). Most of these adverse reactions were mild to moderate in intensity.

 Figure 1 illustrates that the proportion of patients achieving a given level of serum phosphorus lowering is comparable between the two treatment groups. For example, about half the patients in each group had a decrease of at least 0.65 mmol/L at endpoint. Successful control of serum phosphorus in chronic kidney disease patients is multifactorial including reduction of dietary phosphate intake, removal of phosphate with dialysis and inhibition of intestinal phosphate absorption with phosphate binders. As seen in Figure 2, some of the patients in GTC-36-301 did not respond to sevelamer hydrochloride treatment. Not all patients achieve phosphorus control with sevelamer hydrochloride alone, especially at the doses administered in this study (average actual daily dose 4.3 g/day). Later studies which employed higher doses of sevelamer hydrochloride (i.e. GTC-49-301-average actual daily dose 6.5 g/day) had a better rate of phosphorus response.
Figure 1 illustrates that the proportion of patients achieving a given level of serum phosphorus lowering is comparable between the two treatment groups. For example, about half the patients in each group had a decrease of at least 0.65 mmol/L at endpoint. Successful control of serum phosphorus in chronic kidney disease patients is multifactorial including reduction of dietary phosphate intake, removal of phosphate with dialysis and inhibition of intestinal phosphate absorption with phosphate binders. As seen in Figure 2, some of the patients in GTC-36-301 did not respond to sevelamer hydrochloride treatment. Not all patients achieve phosphorus control with sevelamer hydrochloride alone, especially at the doses administered in this study (average actual daily dose 4.3 g/day). Later studies which employed higher doses of sevelamer hydrochloride (i.e. GTC-49-301-average actual daily dose 6.5 g/day) had a better rate of phosphorus response. Average daily consumption at the end of treatment was 4.9 g sevelamer hydrochloride (range of 0.0 to 12.6 g) and 5.0 g of calcium acetate (range of 0.0 to 17.8 g). During calcium acetate treatment, 22% of patients developed serum calcium ≥ 2.75 mmol/L on at least one occasion versus 5% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium acetate.
Average daily consumption at the end of treatment was 4.9 g sevelamer hydrochloride (range of 0.0 to 12.6 g) and 5.0 g of calcium acetate (range of 0.0 to 17.8 g). During calcium acetate treatment, 22% of patients developed serum calcium ≥ 2.75 mmol/L on at least one occasion versus 5% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium acetate. Figure 2, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment.
Figure 2, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment. Average daily consumption at the end of the treatment was 6.5 g of sevelamer hydrochloride (range of 0.8 to 13 g) or approximately eight 800 mg tablets (range of 1 to 16 tablets), 4.6 g of calcium acetate (range of 0.7 to 9.5 g) and 3.9 g of calcium carbonate treatment, 34% of patients in the calcium group developed serum calcium corrected for albumin ≥ 2.75 mmol/L on at least one occasion versus 7% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium salts.
Average daily consumption at the end of the treatment was 6.5 g of sevelamer hydrochloride (range of 0.8 to 13 g) or approximately eight 800 mg tablets (range of 1 to 16 tablets), 4.6 g of calcium acetate (range of 0.7 to 9.5 g) and 3.9 g of calcium carbonate treatment, 34% of patients in the calcium group developed serum calcium corrected for albumin ≥ 2.75 mmol/L on at least one occasion versus 7% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium salts.

 The primary amine groups shown in the structure are derived directly from poly (allylamine hydrochloride). The cross-linking groups consist of two secondary amine groups derived from poly (allylamine hydrochloride) and one molecule of epichlorohydrin.
The primary amine groups shown in the structure are derived directly from poly (allylamine hydrochloride). The cross-linking groups consist of two secondary amine groups derived from poly (allylamine hydrochloride) and one molecule of epichlorohydrin.