What is in this leaflet
This leaflet answers some common questions about Sevelamer Lupin.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Sevelamer Lupin against the benefits this medicine is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet. You may need to read it again.
What Sevelamer Lupin is used for
Sevelamer Lupin contains the active substance sevelamer carbonate and is used to treat hyperphosphatemia, a condition caused by too much dietary phosphorus being retained in your body due to a diseased kidney.
Increased levels of serum phosphorus can lead to hard deposits in your body called calcification. These deposits can stiffen your blood vessels and make it harder for blood to be pumped around the body.
Sevelamer Lupin helps to remove excess phosphorus that has built up in your body by binding the phosphorus that is in the food that you eat.
Your doctor, however, may have prescribed Sevelamer Lupin for another purpose.
Ask your doctor if you have any questions about why it has been prescribed for you.
This medicine is only available with a doctor's prescription.
Before you take Sevelamer Lupin
When you must not take it
Do not take Sevelamer Lupin if you have an allergy to:
- any medicine containing sevelamer carbonate (the active ingredient);
- any of the ingredients listed at the end of this leaflet;
- any other similar medicine, such as sevelamer hydrochloride.
Symptoms that may indicate an allergic reaction include:
- shortness of breath;
- wheezing or difficulty breathing;
- swelling of the face, lips, tongue or other parts of the body;
- rash, itching or hives on the skin.
Tell your doctor if you are experiencing any of the above symptoms.
Do not take Sevelamer Lupin if you:
- have hypophosphatemia, a condition where you do not have enough phosphorus in your body;
- have a bowel obstruction.
Sevelamer Lupin should not be used after the expiry date (EXP) printed on the pack. If you take this medicine after the expiry date it may have no effect at all, or worse, an unexpected effect.
Sevelamer Lupin should not be used if the packaging is torn or shows signs of tampering.
Do not give Sevelamer Lupin to children. The safety and efficacy of Sevelamer Lupin in children under the age of 18 years as not been established.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor or pharmacist if you have:
- allergies to any other medicines or substances such as foods, preservatives or dyes;
- swallowing problems;
- severe constipation;
- problems with movement in your stomach and bowel;
- active inflammation of the bowel;
- undergone major surgery on your stomach or bowel;
- you have or have had any other medical conditions, including a bowel obstruction or hypophosphatemia;
- thyroid problems;
- If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby.
Tell your doctor about any of the above before you take Sevelamer Lupin.
Additional Information
Due to either your kidney condition or your dialysis treatment you may:
- Develop low or high levels of calcium in your blood. Since Sevelamer Lupin does not contain calcium your doctor might prescribe additional calcium tablets.
- Have a low amount of vitamin D in your blood. Therefore, your doctor may monitor the levels of vitamin D in your blood and prescribe additional vitamin D as necessary. If you do not take multivitamin supplements you may also develop low levels of vitamins A, E, K and folic acid in your blood and therefore your doctor may monitor these levels and prescribe supplemental vitamins as necessary.
- Develop peritonitis (infection of your abdominal fluid) associated with your peritoneal dialysis. This risk can be reduced by careful adherence to sterile techniques during bag changes. You should tell your doctor immediately if you experience any new signs or symptoms of abdominal distress, abdominal swelling, abdominal tenderness, constipation, fever, chills, nausea or vomiting.
Taking other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines (including vaccinations), medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may be affected by Sevelamer Lupin or may affect how well Sevelamer Lupin works. You may need different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
The effects of medicines such as cyclosporin, mycophenolate mofetil and tacrolimus (medicines used to suppress the immune system) may be reduced by Sevelamer Lupin. Your doctor will advise you if you are taking these medicines.
Sevelamer Lupin should not be taken at the same time as ciprofloxacin (an antibiotic).
Thyroid hormone deficiency may uncommonly be observed in certain people taking levothyroxine (used to treat low thyroid hormone levels) and Sevelamer Lupin. Therefore, your doctor may monitor the levels of thyroid stimulating hormone in your blood more closely.
If you are taking medicines for heart rhythm problems or for epilepsy, you should consult your doctor before taking Sevelamer Lupin.
How to take Sevelamer Lupin
How much to take
The recommended starting dose of Sevelamer Lupin is 2.4 g to 4.8 g per day to be taken over 3 meals.
For tablets, this means one to two 800 mg tablets with each meal three times a day.
The dose will depend on your serum phosphorus level.
Follow all directions given to you by your doctor or pharmacist carefully. These directions may differ from the information contained in this leaflet.
How to take it
Swallow Sevelamer Lupin tablets whole with a glass of water. Do not crush, chew or break into pieces.
If you are having difficulty swallowing Sevelamer Lupin tablets, speak to you doctor.
How long to take it
Sevelamer Lupin helps lower your dietary phosphate. It does not cure your condition. Therefore, you must continue to take it as directed by your doctor if you expect to lower your phosphate level and keep it down.
You may have to take phosphate-lowering medicines for the rest of your life. If you stop taking Sevelamer Lupin, your phosphate levels may rise again. It is important to keep taking your medicines even if you feel well.
Do not stop taking Sevelamer Lupin. Your doctor may need to change the dose of your other medicines if you stop taking Sevelamer Lupin, so you should only stop when your treating physician tells you to.
If you forget to take it
If you miss a dose, do not take an extra dose to make up for the one you missed. Take the next dose at the usual time with your meal.
It is important to take Sevelamer Lupin as prescribed by your doctor.
If you take too much (overdose)
Immediately telephone your treating physicians or Poisons Information Centre [Australia telephone 13 11 26; in New Zealand telephone 0800 POISON or 0800 764 766], or go to accident and emergency at your nearest hospital, if you think that you or anyone else may have taken too much Sevelamer Lupin.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention. Keep telephone numbers of these places handy.
While you are taking Sevelamer Lupin
Things you must do
If you become pregnant while you are taking Sevelamer Lupin, tell your doctor.
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking Sevelamer Lupin.
Be sure to keep all your doctor's appointments so your progress can be checked.
Your doctor will check your progress and monitor your phosphorus levels from time to time. This helps to ensure you are getting the right dose of Sevelamer Lupin.
Things you must not do
Do not give Sevelamer Lupin to anyone else, even if they have the same condition as you.
Do not use Sevelamer Lupin to treat any other complaints unless your doctor tells you to.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Side effects
Tell your doctor, pharmacist or nurse as soon as possible if you do not feel well while you are taking Sevelamer Lupin.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
If any of the following happen after you have taken Sevelamer Lupin tell your doctor or pharmacist:
- vomiting;
- nausea;
- constipation;
- diarrhoea;
- flatulence;
- indigestion;
- abdominal pain.
These side effects are usually mild.
Tell your doctor immediately if you notice any of the following symptoms:
- rash, itching or hives on the skin;
- severe constipation.
If any of the following happen, tell your doctor immediately or go to the Accident and Emergency at your nearest hospital:
- shortness of breath;
- wheezing or difficulty in breathing;
- swelling of the face, lips, tongue or other parts of the body.
Other side effects not listed above may occur in some patients. Tell your doctor if you notice anything making you feel unwell when you are taking, or soon after you have finished taking Sevelamer Lupin.
After taking Sevelamer Lupin
Storage
Keep Sevelamer Lupin in a cool dry place where the temperature stays below 25°C.
Do not put Sevelamer Lupin in the refrigerator.
Do not put it in the bathroom or near the sink.
Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines.
Keep Sevelamer Lupin where young children cannot reach it. A locked cupboard at least one and a half meters above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking Sevelamer Lupin or your Sevelamer Lupin tablets have passed their expiry date, ask your pharmacist what to do with any tablet that is left over.
Product description
What it looks like
Sevelamer Lupin 800 mg is available as tablets.
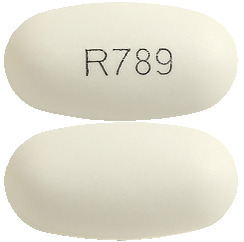
Sevelamer Lupin 800 mg tablets are white to off-white, oval, film- coated tablets imprinted with ‘R789’ on one side and are plain on the other side.
Ingredients
Active ingredient:
- sevelamer carbonate
Other ingredients:
- mannitol;
- crospovidone;
- zinc stearate;
- hyprolose;
- silicon dioxide;
- polyvinyl alcohol;
- purified talc;
- lecithin;
- xanthan gum;
- purified water.
Australian Registration Numbers
Sevelamer Lupin 800 mg tablet: AUST R 286651
Distributor
Generic Health Pty Ltd
Suite 2, Level 2
19-23 Prospect Street
Box Hill, VIC, 3128
Australia
Email: [email protected]
Telephone: +61 3 9809 7900
Website: www.generichealth.com.au
This leaflet was prepared in January 2020.
Published by MIMS June 2020

 For patients previously on calcium based phosphate binders, Table 2 provides guidance on switching to sevelamer carbonate. Serum phosphorus levels should be monitored to ensure optimal daily doses.
For patients previously on calcium based phosphate binders, Table 2 provides guidance on switching to sevelamer carbonate. Serum phosphorus levels should be monitored to ensure optimal daily doses.
 In clinical practice, treatment will be continuous based on the need to control serum phosphorus levels and the daily dose is expected to be an average of approximately 6 g per day.
In clinical practice, treatment will be continuous based on the need to control serum phosphorus levels and the daily dose is expected to be an average of approximately 6 g per day. The most frequently occurring (≥ 2% of patients) undesirable effects possibly or probably related to sevelamer were mainly in the gastrointestinal disorders system organ class (Table 5). Most of these adverse reactions were mild to moderate in intensity.
The most frequently occurring (≥ 2% of patients) undesirable effects possibly or probably related to sevelamer were mainly in the gastrointestinal disorders system organ class (Table 5). Most of these adverse reactions were mild to moderate in intensity.

 Figure 1 illustrates that the proportion of patients achieving a given level of serum phosphorus lowering is comparable between the two treatment groups. For example, about half the patients in each group had a decrease of at least 0.65 mmol/L at endpoint. Successful control of serum phosphorus in chronic kidney disease patients is multifactorial including reduction of dietary phosphate intake, removal of phosphate with dialysis and inhibition of intestinal phosphate absorption with phosphate binders. As seen in Figure 1, some of the patients in GTC-36-301 did not respond to sevelamer hydrochloride treatment. Not all patients achieve phosphorus control with sevelamer hydrochloride alone, especially at the doses administered in this study (average actual daily dose 4.3 g/day). Later studies which employed higher doses of sevelamer hydrochloride (i.e. GTC-49-301-average actual daily dose 6.5 g/day) had a better rate of phosphorus response.
Figure 1 illustrates that the proportion of patients achieving a given level of serum phosphorus lowering is comparable between the two treatment groups. For example, about half the patients in each group had a decrease of at least 0.65 mmol/L at endpoint. Successful control of serum phosphorus in chronic kidney disease patients is multifactorial including reduction of dietary phosphate intake, removal of phosphate with dialysis and inhibition of intestinal phosphate absorption with phosphate binders. As seen in Figure 1, some of the patients in GTC-36-301 did not respond to sevelamer hydrochloride treatment. Not all patients achieve phosphorus control with sevelamer hydrochloride alone, especially at the doses administered in this study (average actual daily dose 4.3 g/day). Later studies which employed higher doses of sevelamer hydrochloride (i.e. GTC-49-301-average actual daily dose 6.5 g/day) had a better rate of phosphorus response. Average daily consumption at the end of treatment was 4.9 g sevelamer hydrochloride (range of 0.0 to 12.6 g) and 5.0 g of calcium acetate (range of 0.0 to 17.8 g). During calcium acetate treatment, 22% of patients developed serum calcium ≥ 2.75 mmol/L on at least one occasion versus 5% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium acetate.
Average daily consumption at the end of treatment was 4.9 g sevelamer hydrochloride (range of 0.0 to 12.6 g) and 5.0 g of calcium acetate (range of 0.0 to 17.8 g). During calcium acetate treatment, 22% of patients developed serum calcium ≥ 2.75 mmol/L on at least one occasion versus 5% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium acetate. Figure 2, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment.
Figure 2, a plot of the phosphorus change from baseline for the completers, illustrates the durability of response for patients who are able to remain on treatment. Average daily consumption at the end of the treatment was 6.5 g of sevelamer hydrochloride (range of 0.8 to 13 g) or approximately eight 800 mg tablets (range of 1 to 16 tablets), 4.6 g of calcium acetate (range of 0.7 to 9.5 g) and 3.9 g of calcium carbonate treatment, 34% of patients in the calcium group developed serum calcium corrected for albumin ≥ 2.75 mmol/L on at least one occasion versus 7% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium salts.
Average daily consumption at the end of the treatment was 6.5 g of sevelamer hydrochloride (range of 0.8 to 13 g) or approximately eight 800 mg tablets (range of 1 to 16 tablets), 4.6 g of calcium acetate (range of 0.7 to 9.5 g) and 3.9 g of calcium carbonate treatment, 34% of patients in the calcium group developed serum calcium corrected for albumin ≥ 2.75 mmol/L on at least one occasion versus 7% for sevelamer hydrochloride (p < 0.05). Thus the risk of developing hypercalcaemia is less with sevelamer hydrochloride compared to calcium salts.

 Chemical Name: poly (allylamine-co-N,N'-diallyl-1,3-diamino-2-hydroxypropane) carbonate salt.
Chemical Name: poly (allylamine-co-N,N'-diallyl-1,3-diamino-2-hydroxypropane) carbonate salt.