What is in this leaflet
This leaflet answers some common questions about Sildenafil Sandoz.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
It should only be used under strict medical supervision.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Sildenafil Sandoz is used for
This medicine is used to treat erectile dysfunction, more commonly known as impotence in men. This is when a man cannot get, or keep a hard erect penis suitable for sexual activity.
It contains the active ingredient sildenafil. Sildenafil belongs to a group of medicines called phosphodiesterase type 5 inhibitors.
It works by relaxing the blood vessels in your penis when you are sexually excited. This allows blood to flow into your penis, allowing you to get an erection in the natural way.
Sildenafil Sandoz will work only if you are sexually excited. It will not increase your sex drive.
Sildenafil Sandoz is not for use in women.
This medicine is available only with a doctor's prescription.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
Before you take Sildenafil Sandoz
You must not take Sildenafil Sandoz if you are taking any nitrates or nitrite medications. It may lead to a severe drop in blood pressure, which may be difficult to treat.
Because sexual activity may place a strain on your heart, your doctor will need to check whether you are fit enough to take Sildenafil Sandoz.
When you must not take it
Do not take this medicine if you have an allergy to:
- sildenafil, the active ingredient, or to any of the other ingredient listed at the end of this leaflet under Product Description
- any other similar medicines.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take Sildenafil Sandoz if:
- you are being treated for angina (chest pain) or other heart conditions with certain medicines called nitrates.
Nitrates medicines include glyceryl trinitrate (also called nitroglycerin).
Common tradenames for glyceryl trinitrate tablets include Anginine and Lycinate.
Common trade names for glyceryl trinitrate patches include Nitro-Dur, Transiderm-Nitro, Nitroderm TTS and Minitran.Common trade names for glyceryl trinitrate sprays include Nitrolingual and Nitronal.
Trade names for glyceryl trinitrate injections include Glyceryl Trinitrate Concentrate and Glyceryl Trinitrate.
Common tradenames for other nitrate preparations include Imdur Durules, Monodur Durules, Sorbidin, Isordil, Imtrate, Duride, Isomonit, Ikorel and Sodium Nitroprusside.
There may be other tradenames not listed here.
- you are taking guanylate cyclase stimulators (GCS), such as Adepmas (riociguat).
GCS is a type of medicine used to treat high blood pressure in the blood vessels in the lungs caused by blood clots in the lungs (chronic thromboembolic pulmonary hypertension, CTEPH) or narrowing of the vessels that carry blood from the heart to the lungs (pulmonary arterial hypertension or PAH).
- you are using amyl nitrite
- you have heart or blood vessel problems that make sexual intercourse inadvisable
- you have suffered a heart attack or stroke in the last six months
- you have severe liver problems
- your blood pressure is unusually high or low or is not effectively treated
- you have loss of vision in one or both eyes from an eye disease called non-arteritic anterior ischaemic optic neuropathy (NAION)
- you have an eye disease called retinitis pigmentosa
- the packaging is torn or shows signs of tampering, or if it is after the expiry date printed on the pack.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines or any other substances such as foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- heart or blood vessel problems
- sudden loss of eyesight in one or both eyes
- diabetes, especially if you also have eye problems
- kidney or liver problems
- leukaemia (cancer of the blood cells)
- multiple myeloma (a cancer of the bone marrow)
- any disease or deformity of your penis
- any bleeding disorder such as haemophilia
- stomach ulcers
- a disease of the blood called sickle cell anaemia
- colour vision problems
- previously experienced sudden decrease or loss of hearing
- any other medical conditions.
Tell your doctor if you are receiving any other treatment for impotence.
Tell your doctor if you are taking any medicines to treat high blood pressure in the vessels of the lungs (pulmonary arterial hypertension) including Tracleer (bosentan) or Revatio which also contains sildenafil.
If you have not told your doctor or pharmacist about any of the above, tell them before you start taking Sildenafil Sandoz.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Do not take Sildenafil Sandoz if you are using nitrate medicines for angina.
Do not take Sildenafil Sandoz if you are taking guanylate cyclase stimulators (GCS), such as Adepmas (riociguat).
Some medicines and Sildenafil Sandoz may interfere with each other. These include:
- cimetidine, a medicine used to treat ulcers
- some medicines used to treat fungal infections including ketoconazole and itraconazole
- some antibiotics including erythromycin and rifampicin
- some protease inhibitors such as ritonavir and saquinavir for the treatment of HIV infection
- medicines called alpha-blockers. These are used treat high blood pressure or prostate problems
- Tracleer (bosentan), a medicine used to treat high blood pressure in the vessels of the lungs.
These medicines may be affected by Sildenafil Sandoz or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
Ask your doctor or pharmacist, if you are not sure if you are taking any of these medicines.
How to take Sildenafil Sandoz
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the pack, ask your doctor or pharmacist for help.
How much to take
Your doctor will decide the correct dose for you depending on your condition and response.
This can be one 25mg tablet a day or one 50mg tablet a day or one 100mg tablet a day.
Do not take more than one dose of Sildenafil Sandoz a day.
How to take it
Swallow the tablet with a full glass of water.
Sildenafil Sandoz tablets have a breaking notch and may be broken if necessary.
When to take Sildenafil Sandoz
Take your dose of Sildenafil Sandoz about one hour before you intend to have sex. The amount of time Sildenafil Sandoz takes to start working varies from person to person, but it normally takes between half an hour and one hour.
You may find that Sildenafil Sandoz takes longer to work if you take it with a heavy meal.
Sildenafil Sandoz will work only if you are sexually excited.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much Sildenafil Sandoz. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much Sildenafil Sandoz, you are more likely to experience side effects.
While you are taking Sildenafil Sandoz
Things you must do
Stop taking Sildenafil Sandoz if you have a loss of eyesight in one or both eyes, experience loss of hearing or have an erection that persists more than 4 hours. Seek medical attention urgently.
If Sildenafil Sandoz does not help you get an erection or if your erection does not last long enough to complete sexual intercourse, tell your doctor. In these cases, your doctor may decide that you need a higher dose.
If you are about to start taking any new medicines, especially nitrates or Adepmas (riociguat), tell your doctor and pharmacist that you are taking Sildenafil Sandoz. See "Before you take Sildenafil Sandoz" for a list of common nitrate medications.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
Things you must not do
Do not use the drug amyl nitrite (sometimes called "poppers") while you are taking Sildenafil Sandoz.
If you get an angina attack, do not take nitrate medicines to relieve the pain, but instead tell your doctor immediately. Make sure your doctor knows you are taking Sildenafil Sandoz.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful drinking alcohol while taking Sildenafil Sandoz. Drinking alcohol can temporarily impair the ability to get an erection. Do not drink large amounts of alcohol before sexual activity.
If you experience changes in vision, or dizziness, when taking Sildenafil Sandoz, you should not drive or operate machinery.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Sildenafil Sandoz.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- headache
- dizziness
- flushing
- hot flushes
- indigestion
- heartburn
- nasal congestion
- sinus congestion
- swelling of your nose
- diarrhoea
- rash
- dry mouth or dry throat
- dry nose
- dry eye
- tightness in your throat
- feeling hot or irritable
- redness in your mouth or tongue.
Tell your doctor as soon as possible if you notice any of the following:
- unusual heart beat
- urinary tract infection (stinging or burning urine, more frequent need to pass urine)
- blood in the urine
- persistent headache or fainting
- bleeding from the nosepain or tingling in your hands, toes or feet
- decreased sensitivity or numbness in your mouth
- irritation or feeling of having something in the eye
- swollen or puffy eye(s)
- fatigue, pain in or around the eyes
- ''red eye'' due to swollen blood vessels in the white part of the eye and in the eyelids.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- signs of allergy such as shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts
- chest pain
- increased heart rate
- sudden decrease or loss of hearing
- seizures, fits or convulsions
- your erection is increased, painful or persists for longer than usual. If your erection continues for four hours, or sooner if there is pain, you should seek medical attention urgently
- rarely men have lost eyesight sometimes after taking medicines to treat erectile dysfunction (known as impotence). It is not known at this time if Sildenafil Sandoz causes this. If you lose eyesight in one or both eyes or experience changes in vision such as blurring, a blue colour to your vision or a greater awareness of light, seek medical attention urgently
- changes to your normal vision such as:
- red or yellow colour tinges to your vision or colourless objects appear coloured
- you see a halo around lights, sparks or lights when your eyes are closed.
This is not a complete list of all possible side effects. Others may occur in some people and there may be side effects not yet known.
If you notice any other symptoms that worry you, check with your doctor.
Ask your doctor or pharmacist if you don't understand anything in this list.
After using Sildenafil Sandoz
Storage
Keep your medicine in the original container. If you take it out of its original container it may not keep well.
Keep your medicine in a cool dry place where the temperature stays below 30°C. Do not store Sildenafil Sandoz or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
Sildenafil Sandoz tablets are available in three strengths:
Sildenafil Sandoz 25mg - light blue, slightly dotted, round tablets, with a breaking notch and marked "25". Available in blisters of 4 tablets.
Sildenafil Sandoz 50mg - light blue, slightly dotted, round tablets, with a cross breaking notch and marked "50". Available in blisters of 4 and 12 tablets.
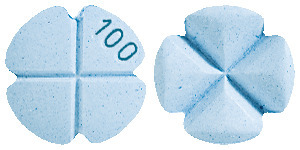
Sildenafil Sandoz 100mg - light blue, slightly dotted, round tablets, with a cross breaking notch and marked "100". Available in blisters of 4 and 12 tablets.
Ingredients
Active ingredients:
- Sildenafil Sandoz 25mg - 25mg sildenafil (as citrate)
- Sildenafil Sandoz 50mg - 50mg sildenafil (as citrate)
- Sildenafil Sandoz 100mg - 100mg sildenafil (as citrate)
Inactive ingredients:
- microcrystalline cellulose
- calcium hydrogen phosphate anhydrous
- copovidone
- croscarmellose sodium
- magnesium stearate
- sodium saccharin
- indigo carmine (E132) aluminium lake.
This medicine does not contain lactose, gluten, tartrazine or any other azo dyes.
Supplier
Sildenafil Sandoz is supplied in Australia by:
Sandoz Pty Ltd
ABN 60 075 449 553
54 Waterloo Road
Macquarie Park NSW 2113
Tel: 1800 726 369
This leaflet was revised in August 2017.
Australian Register Number(s)
Sildenafil Sandoz 25mg tablets:
AUST R 148570 (blisters)
Sildenafil Sandoz 50mg tablets:
AUST R 148567 (blisters)
Sildenafil Sandoz 100mg tablets:
AUST R 148568 (blisters)
Published by MIMS November 2017



 Other adverse reactions occurred at a rate of > 2%, but equally commonly on placebo: respiratory tract infection, back pain, flu syndrome and arthralgia.
Other adverse reactions occurred at a rate of > 2%, but equally commonly on placebo: respiratory tract infection, back pain, flu syndrome and arthralgia.
