What is in this leaflet
This leaflet answers some common questions about STELAX.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking STELAX against the benefits they expect it will have for you.
Talk to your doctor or pharmacist if you have any concerns about taking this medicine.
Keep this leaflet with your medicine. You may need to read it again.
What STELAX is used for
STELAX is used to treat muscle spasms that occur in various illnesses such as multiple sclerosis and diseases and injuries of the spinal cord.
STELAX belongs to a group of medicines called muscle relaxants. These medicines reduce excess tension in your muscles that cause spasms and pain.
Reducing these spasms may help to increase your mobility and make everyday activities easier to manage.
Your doctor may have prescribed this medicine for another reason. Ask your doctor if you have any questions about why it has been prescribed for you.
It is available only with a doctor's prescription.
Before you take it
When you must not take it
Do not take STELAX if you are allergic to medicines containing baclofen or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
Do not take it if the expiry date (Exp.) printed on the pack has passed.
Do not take it if the packaging shows signs of tampering or the tablets do not look quite right.
Before you start to take it
Tell your doctor if you are pregnant or intend to become pregnant or you are breastfeeding. There is very little information on the use of this medicine in pregnancy or while breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you have to take STELAX during pregnancy your baby may have convulsions and other symptoms related to sudden discontinuation of the medicine just after delivery.
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- a mental illness
- Parkinson’s disease
- Seizures (fits) from any cause
- stiffness and restriction of movement in a group of muscles
- stomach ulcers
- stroke or other blood circulation disease
- heart disease
- kidney disease
- liver disease
- lung problems which make breathing difficult
- diabetes
- porphyria, a rare disturbance in the production of porphyrin, a pigment important for liver function and blood formation
- history of alcoholism, drink alcohol to excess or you have a history of drug abuse or dependence. Some people being treated with baclofen have had thoughts of harming or killing themselves or have tried to kill themselves. Most of these people also had depression, had been using alcohol excessively or were prone to having thoughts of killing themselves. If you have thoughts of harming or killing yourself at any time, speak to your doctor straightaway or go to a hospital. Also, ask a relative or close friend to tell you if they are worried about any changes in your behaviour and ask them to read this leaflet.
- high blood pressure
Your doctor may want to take special precautions if you have any of the above conditions. These precautions may include additional tests during or prior to taking STELAX.
If you have not told your doctor about any of the above, tell them before you start taking STELAX.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may be affected by STELAX or may affect how well it works. These include:
- any medicine that tends to make you sleepy, such as medicines used to help you sleep or calm you down, pain relievers and medicines for colds or allergies
- medicines used to treat mood disorders, including tricyclic antidepressants, lithium, and monoamine oxidase inhibitors (MAOIs)
- medicines used to treat diabetes
- medicines used to treat high blood pressure
- medicines used to treat Parkinson’s disease, including selegiline and levodopa and carbidopa.
You may need to take different amounts of your medicines or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take it
Follow all directions given to you by your doctor and pharmacist carefully. These directions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Treatment with STELAX is usually started in hospital with a small dose which is gradually increased to a dose that has the best result for you.
Your doctor will tell you how many tablets you need to take each day and when to take them.
If you are under the age of 16 or over 65 or you have kidney disease, your doctor may start you on a lower dose and increase it more gradually to prevent unwanted side effects.
Follow all directions given to you by your doctor and pharmacist carefully.
How to take it
Take your medicine during or immediately after food with a little glass of water. This will lessen the chance of a stomach upset.
STELAX is usually taken in at least 3 divided doses throughout the day. But your doctor may tell you to take it more or less often, depending on your situation.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
How long to take it for
Continue taking STELAX for as long as your doctor recommends. Your doctor will check your progress to make sure the medicine is working and will discuss with you how long your treatment should continue.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) or go to Accident and Emergency at the nearest hospital, if you think you or anyone else may have taken too much STELAX. Do this even if there are no signs of discomfort or poisoning.
The main symptoms of overdose are: drowsiness, breathing difficulties, consciousness disorders and being unconscious (coma).
Other symptoms may include: feeling confused, hallucinations, agitation, convulsions, blurred vision, unusual muscle slackness, sudden contraction of the muscles, poor or absent reflexes, high or low blood pressure, slow, fast or irregular heartbeat, low body temperature, nausea, vomiting, diarrhoea or excessive salivation, trouble breathing during sleep (sleep apnoea), pain in muscles, fever and dark urine (rhabdomyolysis).
If you have kidney disease and have accidentally taken more tablets than your doctor has prescribed, you may experience neurological symptoms of overdose (e.g. drowsiness, feeling confused, hallucinations).
While you are taking it
Things you must do
If you become pregnant while taking STELAX, tell your doctor immediately. Your doctor can discuss with you the risks of taking it while you are pregnant.
Be sure to keep all of your doctor's appointments so that your progress can be checked. To help prevent unwanted side effects from happening, your doctor may want to do some tests from time to time during the course of your treatment.
If your muscle spasms come back, tell your doctor.
Your doctor may be able to change the dose of STELAX to make it work better for you.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking STELAX.
Tell all the doctors, dentists and pharmacists who are treating you that you are taking this medicine.
Things you must not do
Do not stop taking it suddenly. This medicine is not habit-forming but stopping it suddenly may bring on severe spasms and other unwanted symptoms, such as nervousness, feeling confused, hallucinations, abnormal thinking or behaviour, convulsions, uncontrollable twitching, jerking or writhing movements, fast heartbeat, high body temperature, pain in muscles, fever and dark urine. The excessive stiffness (spasms) in your muscles may also get worse.
If STELAX must be stopped, your doctor will reduce the dose gradually over a period of 1 to 2 weeks so that these unwanted effects are avoided.
Do not use STELAX to treat any other complaints unless your doctor tells you to.
Do not give this medicine to anyone else, even if their symptoms seem to be similar to yours.
Things to be careful of
Be careful driving or operating machinery or doing jobs that require you to be alert while you are taking STELAX until you know how it affects you.
This medicine may cause sleepiness and decreased alertness in some people, especially at the start of treatment.
Be careful when drinking alcohol while taking this medicine. The combination may make you feel more sleepy and less alert than usual.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking STELAX. Like all other medicines, it may have unwanted side effects in some people. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Side effects happen mainly at the start of treatment or if the dose is too high or is increased too rapidly. They can often be relieved by lowering the dose.
If you are over 65 years of age, you may have an increased chance of getting side effects.
As people grow older, they are more likely to get side effects from medicines.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- daytime sleepiness or drowsiness
- lack of energy, tiredness
- dizziness, light-headedness
- spinning sensation (vertigo)
- mental confusion
- headache
- difficulty sleeping, nightmares
- feeling sick (nausea), vomiting or retching
- constipation, stomach cramps, diarrhoea
- loss of appetite
- stuffy or blocked nose
- dry mouth
- change in sense of taste
- misuse, abuse and dependence
- suicide or suicide attempts
- numbness or tingling in the hands or feet
- muscle weakness, spasms or pain
- problems with co-ordination or balance
- swelling of ankles due to fluid build-up
- blurred or double vision
- ringing in the ears
- frequent urination or bed wetting
- excessive sweating
- weight gain
- increased blood sugar
- low body temperature
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- signs of allergy such as rash, itching or hives on the skin; swelling of the face, lips tongue or other parts of the body; shortness of breath or wheezing.
- slow or difficult breathing
- irregular heartbeat (fast or slow)
- chest pain
- uncontrolled muscle spasms affecting the eyes, head, neck or body
- fainting or seizures (fits)
- depression or other severe mood changes or mental changes
- hallucinations (hearing or seeing things that are not there)
- inability to urinate, pain when urinating, blood in the urine
- symptoms following sudden discontinuation of the medicine (drug withdrawal syndrome). Refer to “Things you must not do” above.
The above side effects could be serious. You may need medical attention.
Tell your doctor if you notice anything that is making you feel unwell.
Other side effects not listed above may happen in some people.
After using it
Storage
Keep your medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Keep your tablets in the original container until it is time to take them.
Do not store STELAX or any other medicine in the bathroom or near a sink.
Do not leave it in the car or on window sills. Heat and dampness can destroy some medicines. Stelax will keep well if it is cool and dry.
Disposal
If your doctor tells you to stop taking STELAX, or your tablets have passed their expiry date, ask your pharmacist what to do with any that are left over.
Product description
What it looks like
STELAX comes in 2 strengths of tablets:
- STELAX 10 - round off-white tablet marked BL|10 on one side, and plain on the other.
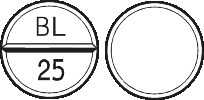
- STELAX 25 - round off-white tablet marked BL|25 on one side, and plain on the other.
Each pack contains 100 tablets.
Ingredients
The active ingredient in STELAX is baclofen.
- each STELAX 10 tablet contains 10 mg of baclofen
- each STELAX 25 tablet contains 25 mg of baclofen.
The tablets also contain:
- microcrystalline cellulose
- maize starch
- povidone
- colloidal anhydrous silica
- magnesium stearate.
The tablets are gluten free.
Sponsor
Arrow Pharma Pty Ltd
15 – 17 Chapel Street
Cremorne VIC 3121
Australian registration numbers:
STELAX 10 - Aust R 92251
STELAX 25 - Aust R 92252
This leaflet was revised in May 2020.
Published by MIMS July 2020

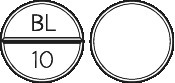
 C10H12ClNO2. Molecular weight: 213.67.
C10H12ClNO2. Molecular weight: 213.67.