What is in this leaflet
This leaflet answers some common questions about STILNOX CR.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What STILNOX CR is used for
STILNOX CR is used to initiate and maintain sleep in those with sleeping difficulties, also called insomnia in patients over 18 years of age. It is not recommended for use for more than 4 weeks at a time.
STILNOX CR has a different chemical structure to other sleeping tablets. STILNOX CR works by binding to special sites in the brain which produce sleep.
Your doctor, however, may prescribe STILNOX CR for another purpose.
Ask your doctor or pharmacist if you have any questions about why it has been prescribed for you.
This medicine is only available with a doctor's prescription.
Before you take STILNOX CR
When you must not take it
Do not take STILNOX CR if you have:
- been drinking alcohol or you believe that you may have alcohol in your bloodstream
- sleep apnoea (a condition where you temporarily stop breathing while you sleep)
- myasthenia gravis (a condition in which the muscles become weak and tire easily)
- severe liver problems
- acute and/or severe lung problems
- previously experienced complex sleep behaviours after taking this medicine including sleep-walking, sleep-driving, and/or engaging in other activities while not fully awake.
Do not take STILNOX CR if you are allergic to it or any of the ingredients listed at the end of this leaflet. Some symptoms of an allergic reaction include skin rash, itching, shortness of breath or swelling of the face, lips or tongue, which may cause difficulty in swallowing or breathing.
Do not give STILNOX CR to a child or adolescent. There is no experience with its use in children or adolescents under 18 years of age.
Talk to your doctor or pharmacist if you have ever had a mental disorder or have abused or have been dependent on alcohol or drugs.
Do not take it after the expiry date (EXP) printed on the pack. If you take it after the expiry date has passed, it may not work as well.
Do not take it if the packaging is damaged or shows signs of tampering.
Before you start to take it
Tell your doctor if you have allergies to any of the ingredients listed at the end of this leaflet.
Contains lactose and sugars.
Tell your doctor if you are pregnant, suspect that you are pregnant or intend to become pregnant. Like most medicines of this kind, STILNOX CR is not recommended to be used during pregnancy. Your doctor will discuss the risks and benefits of taking it if you are pregnant.
Tell your doctor if you are breast-feeding or planning to breast-feed. STILNOX CR can pass into breast milk. Your doctor will discuss the risks and benefits of using it if you are breast-feeding or planning to breast-feed.
Tell your doctor if you have any problems with your breathing or if you often snore while you are asleep.
Tell your doctor if you have ever been addicted to alcohol or any drug or medicine or if you have ever suffered from a mental illness. If you have, you may be at risk of getting into a regular pattern or habit of taking STILNOX CR.
Tell your doctor if you have or have had any medical conditions, especially the following:
- problems with your heart, liver, kidneys or lungs
- epilepsy
- depression
- mental illness, for example, schizophrenia
Tell your doctor if you plan to have surgery.
If you have not told your doctor about any of the above, tell them before you take STILNOX CR.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from your pharmacy, supermarket or health food store.
Some medicines may interfere with STILNOX CR. These include:
- medicines to treat depression, anxiety and mental illness
- medicines to treat epilepsy
- pain relievers
- muscle relaxants
- antihistamines
- St John's Wort (also known as Hypericum), a herbal remedy used to treat depression
- rifampicin and ciprofloxacin, medicines used to treat infections
- ketoconazole, a medicine to treat antifungal infections
These medicines may be affected by STILNOX CR, or may affect how well it works i.e. by increasing drowsiness. This may affect your ability to drive a car or operate dangerous machinery. You may need to use different amounts of your medicine, or take different medicines. Your doctor will advise you.
Your doctor or pharmacist has more information on medicines to be careful with or to avoid while taking STILNOX CR.
How to take STILNOX CR
How much to take
STILNOX CR should only be taken when you are able to get a full night's sleep (7 to 8 hours) before you need to be active again. It should be taken in one dose and not be re-administered during the same night.
The usual adult dose of STILNOX CR is one 12.5 mg tablet taken just before you go to bed.
If you are over 65 years of age the dose is one STILNOX CR 6.25 mg tablet taken just before you go to bed.
If you have a liver problem, the usual recommended dose is one STILNOX CR 6.25 mg tablet.
Your doctor may have prescribed a different dose.
Ask your doctor if you are unsure of the correct dose for you. They will tell you exactly how much to take.
Follow the instructions they give you. If you take the wrong dose, STILNOX CR may not work as well. If you take too much your consciousness may be impaired (see 'Overdose' below).
STILNOX CR should not be given to children or adolescents less than 18 years of age.
How to take it
Swallow the tablet whole with a full glass of water.
Do not crush or chew the tablet. Each STILNOX CR tablet has been especially designed to release the right dose of medicine while you sleep. If you crush, chew or divide STILNOX CR tablets they will not work properly.
When to take it
Take STILNOX CR immediately before you go to bed or while you are in bed. It helps put you to sleep quite quickly. If you take STILNOX CR on an empty stomach it may work more quickly.
If you are not sure when to take it ask your doctor or pharmacist.
How long to take it
Usually, STILNOX CR or any other medicines to treat sleeping disorders should only be used for short periods (e.g. 2 to 4 weeks). Continuous long term use is not recommended unless advised by your doctor.
Ask your doctor or pharmacist if you are not sure how long to take the medicine for.
If you forget to take it
If you forget to take the tablet before you go to bed, and you wake up late in the night or very early in the morning, do not take it. You may have trouble waking at your normal time.
If you are not sure what to do, ask your doctor.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26), or go to Accident and Emergency at your nearest hospital, if you think you or anyone else may have taken too much STILNOX CR.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much STILNOX CR, your consciousness may be impaired, ranging from drowsiness to light coma.
While you are taking STILNOX CR
Things you must do
Tell all the doctors, dentists and pharmacists who are treating you that you are taking STILNOX CR.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking STILNOX CR.
If you plan to have surgery that needs a general anaesthetic, tell your doctor or dentist that you are taking this medicine.
If you become pregnant or suspect that you are pregnant while you are taking this medicine, stop taking it and tell your doctor or pharmacist immediately.
Things you must not do
Do not take more than the recommended dose unless your doctor tells you to. This can increase the risk of side effects.
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not use this medicine to treat any other complaints unless your doctor tells you to.
Do not drink alcohol before or after taking this medicine. This can increase the risk of side effects.
Things to be careful of
Because STILNOX CR will make you sleepy, you should not operate dangerous machinery or drive motor vehicles for 8 hours after you take it. You should also be careful the next morning when you wake up. Make sure you know how you react to STILNOX CR before you drive a car or operate machinery. This is very important if you are taking other drugs that also make you drowsy.
Be careful if you are over 65 and unwell or taking other medicines. You may be more sensitive to some of the side effects of STILNOX CR.
Some patients may be particularly susceptible to the sedative effects of the medication, which may increase the possibility of a fall.
You should not drink alcohol while you are taking STILNOX CR. The effects of alcohol could be made worse while taking STILNOX CR.
Side effects
All medicines have some unwanted side effects. Sometimes they are serious, but most of the time they are not. Your doctor or pharmacist has weighed the risks of using this medicine against the benefits they expect it will have for you.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor as soon as possible if you do not feel well while you are taking STILNOX CR.
It helps most people with insomnia, but it may have unwanted side effects in some people.
Tell your doctor if you notice any of the following and they worry you:
- drowsiness
- dizziness
- headache
- fatigue
- anxiety
- disorientation
- hallucinations
- nightmares
- slowness in thinking and movement
- poor attention and concentration
- memory impairment and loss
- diarrhoea, nausea and vomiting
- constipation
- stomach discomfort, indigestion, wind, frequent bowel movements
- changes in appetite
- aching muscle, muscle weakness or tenderness not caused by exercise
- muscle cramps
- neck and back pain
- influenza or flu-like symptoms, such as high temperature, sore throat, runny nose, cough and chills
- visual disturbances
These are the most common side effects of this medicine.
Less common adverse effects include:
Unexpected changes in behaviour. These have included rage reactions, worsened insomnia, confusion, agitation, hallucinations and other forms of unwanted behaviour.
Alcohol can increase the risk of sleep walking and other related behaviours. These side effects can also occur without the presence of alcohol.
Although these side effects can occur at the usual recommended doses, the risk of these behaviours occurring may also be increased if you take more than the recommended dose.
Some sleep medicines may cause a short-term memory loss. When this occurs, a person may not remember what has happened for several hours after taking the medicine. This is usually not a problem since most people fall asleep after taking the medicine.
Sleep medicines should in most cases, be used only for short periods of time. If your sleep problems continue, consult your doctor.
Some medicines can cause dependence, especially when they are used regularly for longer than a few weeks. People who have been dependent on alcohol or other drugs in the past may have a higher chance of becoming addicted to sleep medicines. If you have been addicted to alcohol or drugs in the past, it is important to tell your doctor before starting STILNOX CR.
If any of the following happen, stop taking this medicine and tell your doctor immediately, or go to Accident and Emergency at your nearest hospital:
- swelling of the face, lips, mouth or throat, which may cause difficulty in swallowing or breathing
- hives
- fainting
- sleep walking, driving motor vehicles and other unusual, and on some occasions dangerous, behaviours whilst apparently asleep. These have also included preparing and eating food, making phone calls or having sexual intercourse. People experiencing these effects have had no memory of the events.
These are very serious side effects. If you have them, you may have had a serious allergic reaction to STILNOX CR. You may need urgent medical attention or hospitalisation.
These side effects are very rare.
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some consumers.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
After taking STILNOX CR
Sometimes when medicines are stopped suddenly, after being used for a long time, withdrawal symptoms may occur. Symptoms of withdrawal may include abdominal and muscle cramps, vomiting and sweating.
In some cases your insomnia may appear worse for a short time which may be accompanied with other reactions including mood changes, anxiety and restlessness; speak to your doctor if this occurs.
Patients taking part in trials have not had any problems when they stopped taking STILNOX CR.
However, let your doctor know if you have any problems when you stop taking STILNOX CR.
If you have any queries about any aspect of your medicine, or any questions regarding the information in this leaflet, discuss them with your doctor or pharmacist.
Storage
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the box or the blister pack they may not keep well.
Keep the medicine in a cool, dry place where the temperature stays below 30°C.
Do not store it or any other medicine in the bathroom, near a sink, or on a windowsill.
Do not leave it in the car. Heat and damp can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking the medicine or it has passed its expiry date, ask your pharmacist what to do with any that are left over.
Return any unused medicine to your pharmacist.
Product description
What it looks like
STILNOX CR 6.25 mg tablets are pink, bi-convex two-layer tablets engraved with ZMR on one side.
STILNOX CR 12.5 mg tablets are blue, bi-convex two-layer tablets engraved with ZMR on one side.
STILNOX CR 6.25 mg tablets are available in boxes of 14 tablets.
STILNOX CR 12.5 mg tablets are available in boxes of 14 tablets.
Ingredients
Each STILNOX CR 6.25 mg tablet contains zolpidem tartrate 6.25 mg as the active ingredient.
Each STILNOX CR 12.5 mg tablet contains zolpidem tartrate 12.5 mg as the active ingredient.
Inactive Ingredients:
- lactose monohydrate
- microcrystalline cellulose
- hypromellose
- sodium starch glycollate type A
- magnesium stearate
- titanium dioxide
- macrogol 3350
- colloidal anhydrous silica
- iron oxide yellow (STILNOX CR 12.5 mg)
- iron oxide red (STILNOX CR 6.25 mg)
- potassium hydrogen tartrate
- indigo carmine
STILNOX CR does not contain gluten.
Sponsor
STILNOX CR is supplied in Australia by:
sanofi-aventis australia pty ltd
12-24 Talavera Road
Macquarie Park NSW 2113
Email: [email protected]
Tel: 1800 818 806
This leaflet was prepared in February 2020.
Australian Register Number(s)
6.25 mg tablets: AUST R 120707
12.5 mg tablets: AUST R 120713
® Registered Trademark
stilnox-cr-ccdsv16-cmiv14-24feb20
Published by MIMS May 2020

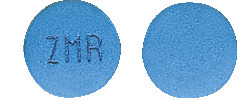
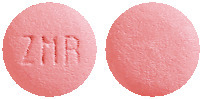
 Table 2 was derived from a pool of three placebo-controlled long-term efficacy trials involving Stilnox (zolpidem tartrate). These trials involved patients with chronic insomnia who were treated for 28 to 35 nights with zolpidem at doses of 5, 10 or 15 mg. Table 2 is limited to data from doses up to and including 10 mg, the highest dose recommended for use. Table 2 includes only adverse events occurring at an incidence of at least 1% for Stilnox patients.
Table 2 was derived from a pool of three placebo-controlled long-term efficacy trials involving Stilnox (zolpidem tartrate). These trials involved patients with chronic insomnia who were treated for 28 to 35 nights with zolpidem at doses of 5, 10 or 15 mg. Table 2 is limited to data from doses up to and including 10 mg, the highest dose recommended for use. Table 2 includes only adverse events occurring at an incidence of at least 1% for Stilnox patients. Table 3 was derived from pooled results of two placebo-controlled efficacy trials involving modified-release zolpidem. These trials involved patients with primary insomnia who were treated for 3 weeks with modified-release zolpidem at doses of 6.25 or 12.5 mg. Table 3 includes only adverse events occurring at an incidence of at least 1% for modified-release zolpidem patients.
Table 3 was derived from pooled results of two placebo-controlled efficacy trials involving modified-release zolpidem. These trials involved patients with primary insomnia who were treated for 3 weeks with modified-release zolpidem at doses of 6.25 or 12.5 mg. Table 3 includes only adverse events occurring at an incidence of at least 1% for modified-release zolpidem patients.
 Elevated liver enzymes, rash, pruritus and urticaria have also been reported.
Elevated liver enzymes, rash, pruritus and urticaria have also been reported. Its chemical name is 2-(4-methylphenyl)- N,N,6-trimethylimidazo [1,2,a] pyridine-3-acetamide hemitartrate.
Its chemical name is 2-(4-methylphenyl)- N,N,6-trimethylimidazo [1,2,a] pyridine-3-acetamide hemitartrate.