Notes
Distributed by Wagner Pharmaceuticals Pty Ltd
1 Name of Medicine
Tamsulosin hydrochloride.
2 Qualitative and Quantitative Composition
Tamsulosin-WGR SR modified release tablets contain the active ingredient tamsulosin hydrochloride.
Each Tamsulosin-WGR SR modified release tablet contains 400 microgram of tamsulosin hydrochloride.
For the full list of excipients, see Section 6.1 List of Excipients.
3 Pharmaceutical Form
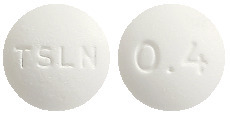
Tamsulosin-WGR SR 400 microgram tablets are white to off-white round tablets with a diameter of approximately 9 mm, debossed with "TSLN" on one side and "0.4" on the other side.
4.1 Therapeutic Indications
For the relief of lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH).
4.2 Dose and Method of Administration
One tablet daily.
The tablet must be swallowed whole and not be broken, crunched or chewed, as this compromises the modified release properties of the tablet for the active ingredient.
Tamsulosin-WGR SR can be taken on an empty stomach, or before, with or after food.
4.3 Contraindications
Hypersensitivity, including drug-induced angioedema, to tamsulosin hydrochloride or any other component of the product.
A history of orthostatic hypotension.
Severe hepatic impairment (Child-Pugh scores > 9).
Severe renal impairment with creatinine clearance of less than 10 mL/min.
Concurrent use of another α1-adrenoceptor inhibitor.
4.4 Special Warnings and Precautions for Use
Syncope and postural hypotension.
Patients beginning treatment with tamsulosin tablets should be cautioned to avoid situations where injury could result should syncope occur. Postural hypotension can occur during treatment with tamsulosin tablets, but rarely results in syncope. However, the patient should be warned of this possibility and advised to sit or lie down if symptoms of hypotension should occur.
Exclusion of prostatic carcinoma and other urological conditions.
Carcinoma of the prostate and other conditions which can cause the same symptoms as benign prostatic hyperplasia should be excluded before starting therapy with tamsulosin tablets. Digital rectal examination and, as considered appropriate, determination of prostate specific antigen should be performed before treatment and at regular intervals afterwards.
Myocardial ischaemia.
Patients with myocardial infarction or angina pectoris within the preceding six months were excluded from the Phase III clinical studies. As a result, the safety of tamsulosin tablets in these patients has not been formally assessed.
Dizziness.
As tamsulosin tablets may cause dizziness, patients should be warned to take care whilst operating machinery or driving.
Intra-operative floppy iris syndrome.
Intra-operative Floppy Iris Syndrome (IFIS) has been observed during cataract and glaucoma surgery in some patients taking or who have previously been treated with α1-adrenoceptor antagonists, including tamsulosin. This variant of small pupil syndrome is characterised by the combination of a flaccid iris that billows in response to intra-operative irrigation currents, progressive intra-operative miosis despite pre-operative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phaco-emulsification incisions.
During pre-operative assessment, ophthalmologists and ophthalmic teams should consider whether patients scheduled for cataract or glaucoma surgery are being, or have been, treated with α1-adrenoceptor antagonists in order to ensure that appropriate measures will be in place to manage IFIS during surgery if it occurs. The patient's ophthalmologist should be prepared for possible modifications to their surgical technique, such as the utilisation of iris hooks, iris dilator rings, or visco-elastic substances. The benefit of stopping α1-adrenoceptor antagonist therapy prior to cataract or glaucoma surgery has not been established.
Sulfa allergy.
Cases of allergic reaction to tamsulosin in patients with a past history of sulphonamide allergy have been reported. If a patient reports a sulfa allergy, caution is warranted when administering tamsulosin tablets.
Use in hepatic impairment.
In a study of patients with moderate hepatic impairment, free tamsulosin levels remained unchanged after treatment with 400 microgram tamsulosin hydrochloride in a modified release capsule formulation when compared to normal subjects. Since the type of formulation will not affect the disposition of tamsulosin no dose adjustment for tamsulosin tablets is expected in patients with mild to moderate hepatic impairment.
Severe hepatic impairment (Child-Pugh scores > 9) is a contraindication, see Section 4.3 Contraindications.
Use in renal impairment.
Severe renal impairment, with creatinine clearance of less than 10 mL/min is a contraindication, as these patients have not been studied, see Section 4.3 Contraindications.
Use in the elderly.
See Section 4.2 Dose and Method of Administration.
Paediatric use.
Tamsulosin-WGR SR is not indicated for use in children.
Other populations.
Tamsulosin-WGR SR is not indicated for use in women.
Effects on laboratory tests.
Interactions with laboratory tests have not been established.4.5 Interactions with Other Medicines and Other Forms of Interactions
Drugs known to interact with tamsulosin.
Concomitant cimetidine leads to a rise in plasma levels of tamsulosin, while furosemide leads to a fall (about 12% following a single 20 mg intravenous dose). However, as levels remain within the normal range, dosage need not be adjusted.
Concurrent administration of tamsulosin with other α1-adrenoceptor antagonists is contraindicated because of the potential for hypotensive effects, see Section 4.3 Contraindications.
Drugs which may interact with tamsulosin.
Tamsulosin binds extensively to plasma proteins and may displace other protein-bound drugs. Clinical trial data are not available.
No interactions at the level of hepatic metabolism have been seen during in vitro studies with liver microsomal fractions (representative of the cytochrome P450-linked drug metabolising enzyme system), involving amitriptyline, salbutamol, glibenclamide and finasteride. Diclofenac and warfarin, however, may increase the elimination rate of tamsulosin.
Drugs which do not interact significantly with tamsulosin.
Tamsulosin did not affect the pharmacokinetics of a single intravenous dose of digoxin 0.5 mg.
No interactions have been seen when tamsulosin hydrochloride was given concomitantly with either atenolol, enalapril, nifedipine or theophylline.
General.
Tamsulosin is metabolised in the liver and may be expected to interact with other hepatically-metabolised drugs. Pharmacokinetic studies in healthy volunteers revealed that concomitant administration with strong inhibitors of CYP3A4 or CYP2D6 may lead to increased exposure to tamsulosin. Concomitant administration with ketoconazole (a known CYP3A4 inhibitor) resulted in an increased Cmax and AUC of tamsulosin. Tamsulosin 400 microgram should not be used in combination with strong inhibitors of CYP3A4 in patients known to be CYP2D6 poor metabolizers. Concomitant administration with paroxetine (a known CYP2D6 inhibitor) resulted in an increased Cmax and AUC of tamsulosin. Tamsulosin should therefore be used with caution in patients who are taking other drugs, particularly those which undergo hepatic metabolism.
Other in vitro findings.
In vitro, neither diazepam nor propranolol, trichlormethiazide, chlormadinone, amitriptyline, diclofenac, glibenclamide, simvastatin and warfarin change the free fraction of tamsulosin in human plasma. Neither does tamsulosin change the free fractions of diazepam, propranolol, trichlormethiazide and chlormadinone.
An in vitro study using human liver microsomal fractions showed no effect of amitriptyline, salbutamol, glibenclamide and finasteride on the rate of disappearance of tamsulosin. The clinical relevance of these findings is uncertain.4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
α-adrenoceptor antagonists are known to reduce male fertility by affecting penile erection, emission and/or ejaculation. In male rats, a severe reduction in male copulation rate and fertility was observed after a single dose or after repeated oral doses of tamsulosin. Spermatogenesis was not affected in the rat studies, and the effect on fertility was reversible. The no effect dose on male rat fertility was associated with plasma tamsulosin levels (AUC) at least 50% of those expected in human males treated with tamsulosin.
Treatment of female rats with tamsulosin caused disruption of the oestrus cycle and a severe reduction in fertility, due to interference of fertilisation with the ova. These effects were shown to be reversible.
(Category B2)
Tamsulosin is intended for use only in males.
Tamsulosin, at oral doses causing maternal toxicity, was not embryotoxic or teratogenic when administered during gestation in rats (doses up to 300 mg/kg/day) or rabbits (doses up to 50 mg/kg/day). However, administration of tamsulosin during the peri-/post-natal period was associated with a higher incidence of stillbirths and reduced pup weight gain after birth. No adverse effects on development or reproductive performance were observed on surviving pups, however, there is some evidence for impairment of offspring reproductive capacity when maternal treatment with tamsulosin is started before pregnancy.
Tamsulosin is intended for use only in males.
In female rats, tamsulosin and/or its metabolites were shown to pass into milk after oral administration of the drug during lactation. The effect on the newborn is not known.4.7 Effects on Ability to Drive and Use Machines
As tamsulosin tablets may cause dizziness, patients should be warned to take care whilst operating machinery or driving.
4.8 Adverse Effects (Undesirable Effects)
Priapism.
Rarely, tamsulosin, like other alpha-1 antagonists, has been associated with priapism (persistent painful penile erection unrelated to sexual activity). Patients should be informed that this reaction is extremely rare, but if not brought to immediate medical attention, can lead to permanent erectile dysfunction.
Abnormal ejaculation.
Patients should be advised on the potential for abnormal ejaculation to occur upon commencement of tamsulosin treatment. Retrograde ejaculation is the most commonly reported abnormal ejaculation event associated with the use of tamsulosin tablets (see Table 1).
Clinical trials.
Table 1 shows the incidence of undesirable effects following 400 microgram tamsulosin tablet treatment. This data is based on a phase 3 clinical study in which there were no relevant differences between the treatment and placebo groups in the percentage of patients reporting at least 1 Treatment Emergent Adverse Event (TEAE). Most TEAEs were of mild or moderate intensity.
The most frequent TEAEs were ejaculation disorders. These are TEAEs that are often associated with α1-AR antagonists.
 The following treatment-related adverse events were reported from clinical trials, where Common is ≥ 1% and < 10%; Uncommon is ≥ 0.1% and < 1%; Rare is ≥ 0.01% and < 0.1%; and Very rare is < 0.01%.
The following treatment-related adverse events were reported from clinical trials, where Common is ≥ 1% and < 10%; Uncommon is ≥ 0.1% and < 1%; Rare is ≥ 0.01% and < 0.1%; and Very rare is < 0.01%.
Cardiac disorders.
Uncommon: palpitations.
Gastro-intestinal disorders.
Uncommon: constipation, diarrhoea, nausea, vomiting.
General disorders.
Uncommon: asthenia.
Nervous system disorders.
Common: dizziness (1.3%), insomnia. Uncommon: headache. Rare: syncope.
Reproductive system disorders.
Common: ejaculation disorder. Very rare: priapism.
Respiratory, thoracic and mediastinal disorders.
Uncommon: rhinitis.
Skin and subcutaneous tissue disorders.
Uncommon: rash, pruritus, urticaria. Rare: angioedema.
Vascular disorders.
Uncommon: postural hypotension.
Post-marketing experience.
The following events have also been reported during the post-marketing period. These events are reported voluntarily from a population of uncertain size; therefore, it is not possible to reliably estimate their frequency.
General disorders.
Chest discomfort that could be caused or associated with other medical conditions such as respiratory conditions or cardiac disease.
Vision disorders.
Blurred vision, vision impairment.
During cataract and glaucoma surgery, a variant of small pupil syndrome known as Intra-operative Floppy Iris Syndrome (IFIS) has been reported in association with α1-adrenoceptor antagonist therapy, see Section 4.4 Special Warnings and Precautions for Use, Intra-operative floppy iris syndrome.
Skin and subcutaneous tissue disorders.
Skin desquamation, dermatitis exfoliative, erythema multiforme, Stevens-Johnson syndrome, photosensitivity reaction.
Respiratory, thoracic and mediastinal disorders.
Epistaxis.
Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at http://www.tga.gov.au/reporting-problems.4.9 Overdose
Acute overdose with 5 mg tamsulosin hydrochloride has been reported. Acute hypotension (systolic blood pressure 70 mmHg), vomiting and diarrhoea were observed, which were treated with fluid replacement and the patient could be discharged the same day.
If acute hypotension occurs after overdosage, cardiovascular support should be given and maintained. Blood pressure can be restored and heart rate brought back to normal by lying the patient down. If this is insufficient then volume expanders and, when necessary, vasopressors could be administered. Renal function should be monitored and general supportive measures applied. Dialysis is unlikely to be of help as tamsulosin is very highly bound to plasma proteins.
Tamsulosin tablet is a modified release formulation. The signs and symptoms of overdose may be delayed or modified from the time of ingestion.
For information on the management of overdose, contact the Poisons Information Centre on 131126 (Australia).
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Mechanism of action.
The tone of the human prostate smooth muscle is maintained primarily by noradrenaline released from adrenergic nerves and stimulating post-junctional α1-adrenoceptors. This provides the rationale for the use of α1-adrenoceptor antagonists for lower urinary tract symptoms associated with benign prostatic hyperplasia (BPH).
Pharmacological studies have established that tamsulosin is a selective, potent and competitive α1-adrenoceptor antagonist and that it has a greater affinity for the α1A-receptor subtype, predominantly present in the human prostate.
α1-adrenoceptor antagonists generally can reduce blood pressure by lowering peripheral resistance. However, no reduction in blood pressure of any clinical significance was observed during studies with tamsulosin.
The binding of tamsulosin to α1-adrenoceptors in the prostate results in relaxation of prostate smooth muscle followed by improvements in urodynamics. Thus, tamsulosin increases maximum urinary flow rate by reducing smooth muscle tension in the prostate and urethra and thereby relieving obstruction.
It also improves the symptoms related to bladder instability and tension of the smooth muscle of the lower urinary tract.
These effects on urinary storage and voiding symptoms are maintained during long-term therapy.
The need for surgery or catheterisation is significantly delayed.
Clinical trials.
The efficacy of tamsulosin tablets has been evaluated in 2 randomised, placebo-controlled studies: the phase 2 dose-response study 617-CL-303 and the phase 3 study 617-CL-307. A total of 2962 patients were studied, of which 560 were treated with 0.4 mg of tamsulosin tablets and 564 were treated with placebo. The remaining subjects were treated with 0.4 mg (capsules), 0.8 mg and 1.2 mg (tablets) doses of tamsulosin hydrochloride.
Inclusion criteria. In both studies the inclusion criteria were male patients aged ≥ 45 years, diagnosed as having lower urinary tract symptoms (LUTS) suggestive of BPH, with voiding/obstructive symptoms (including incomplete emptying of the bladder, intermittency, poor stream or hesitancy), and/or storage/irritative/filling symptoms (including daytime frequency, urgency or nocturia).
These patients had a total International Prostate Symptom Score (I-PSS) of ≥ 13, both at enrolment (Visit 1) and at baseline after the 2-week placebo run-in period (Visit 2). At enrolment, they also had to have a maximum flow rate (Qmax) of ≥ 4.0 mL/s and ≤ 12.0 mL/s, with a voided volume ≥ 120 mL during free flow.
Patients with cardiac ischaemia were excluded from participation in these trials. Safety in such patients has not been formally assessed.
Study 617-CL-303.
Study 617-CL-303 was a multi-center, double-blind, randomised, placebo-controlled, parallel group, dose-response study. In this study, 211 patients received placebo, and 203 patients received 400 microgram of tamsulosin tablets once daily for 12 weeks of the double-blind randomised treatment. The results of study 617-CL-303 are summarised in Table 2.
Study 617-CL-307.
Study 617-CL-307 was a multi-center, double-blind, randomised, placebo and active-controlled, parallel group study. In this study, 353 patients received placebo, and 357 patients received 400 microgram of tamsulosin tablets once daily for 12 weeks of the double-blind randomised treatment. The results of study 617-CL-307 are summarised in Table 3.
The primary efficacy parameter in both studies following 400 microgram tamsulosin treatment was the change from baseline to endpoint in total I-PSS scores. The secondary efficacy analyses contained the changes from baseline in voiding and storage I-PSS sub-scores, and I-PSS Quality of Life scores.
The I-PSS questionnaire was developed and validated by the American Urological Association (I-PSS previously called the AUA Symptom Index) and consisted of 7 questions evaluating the frequency of 7 urinary symptoms. These included 4 voiding symptoms (poor stream, hesitancy, intermittency and incomplete bladder emptying) and 3 storage symptoms (daytime frequency, nocturia and urgency). The patient rated each of the 7 symptoms on a scale of 0-5 of increasing symptom severity. The total score could therefore range from 0-35, the voiding sub-score from 0-20 and the storage sub-score from 0-15. The questionnaire was adopted by the World Health Organization, who added a further question assessing the impact of the urinary symptoms on the Quality of Life. The Quality of Life question asked how the patient would feel about his current level of symptoms for the rest of his life, ranging from 1 (delighted) to 6 (terrible).


5.2 Pharmacokinetic Properties
Absorption.
Tamsulosin is a modified release tablet of the non-ionic gel matrix type. The tamsulosin formulation provides consistent slow release of tamsulosin, which is maintained over the whole pH range encountered in the gastro-intestinal tract, resulting in an adequate exposure, with little fluctuation, over 24 hours.
Tamsulosin administered is absorbed from the intestine. Of the administered dose, approximately 55 to 59% is estimated to be absorbed. The rate and extent of absorption of tamsulosin hydrochloride administered as tamsulosin tablets are only slightly affected by food, but this is unlikely to be clinically significant.
Tamsulosin hydrochloride administered as tamsulosin tablets exhibits near linear pharmacokinetics (plasma concentrations Cmax and AUC vs dose) over the dosage range 0.4 mg through 0.8 mg to 1.2 mg once daily. Steady state is reached by day 4 of multiple dosing. The pharmacokinetics of a 400 microgram once daily dose of tamsulosin hydrochloride as tamsulosin tablets as a single dose under fasted conditions, and steady state under fed and fasted conditions, are shown in Table 4.
 As a result of the modified release characteristic of tamsulosin, the trough concentrations - at steady state, of tamsulosin hydrochloride in plasma amount to approximately 40% of the peak plasma concentrations, under fasted and fed conditions.
As a result of the modified release characteristic of tamsulosin, the trough concentrations - at steady state, of tamsulosin hydrochloride in plasma amount to approximately 40% of the peak plasma concentrations, under fasted and fed conditions.
There is a considerable inter-patient variation in the plasma concentrations of tamsulosin hydrochloride, after both single and multiple dosing.
Distribution.
In man, tamsulosin is about 99% bound to plasma proteins. The volume of distribution is small (about 0.2 L/kg).
Metabolism.
Tamsulosin 400 microgram modified release tablet contains tamsulosin as the R(-) isomer. In humans, there is no in vivo conversion to the less active S(+) isomer. Tamsulosin has a low first pass effect, being metabolised slowly. Most tamsulosin is present in plasma in the form of unchanged drug. Tamsulosin is metabolised in the liver. In vitro results suggest that CYP3A4 and also CYP2D6 are involved in metabolism, with possible minor contributions to tamsulosin metabolism by other CYP isozymes. Inhibition of hepatic drug metabolising enzymes may lead to increased exposure to tamsulosin, see Section 4.4 Special Warnings and Precautions for Use; Section 4.5 Interactions with Other Medicines and Other Forms of Interactions. In rats, tamsulosin was seen to cause minimal induction of microsomal liver enzymes. No dose adjustment is warranted in hepatic insufficiency, see Section 4.3 Contraindications.
None of the metabolites is more active than the original precursor compound.
Excretion.
Tamsulosin and its metabolites are mainly excreted in the urine. The amount excreted as unchanged drug is estimated to be about 4 - 6% of the dose administered as tamsulosin.
No dose adjustment is warranted in renal impairment, see Section 4.3 Contraindications.
5.3 Preclinical Safety Data
Genotoxicity.
In vivo and in vitro genotoxicity studies have been conducted.
Tamsulosin HCI produced no evidence of genotoxic potential in assays for gene mutation (Ames reverse mutation test and mouse lymphoma thymidine kinase assay), chromosomal damage (Chinese hamster ovary cells and mouse micronucleus assay) and other genotoxic effects (unscheduled DNA repair synthesis and in vivo sister chromatid exchange).
Carcinogenicity.
Reproduction toxicity studies in rats and carcinogenicity studies in mice and rats have been conducted.
Oral (dietary) administration of tamsulosin for up to 2 years in rats and mice was associated with an increased incidence of pituitary adenoma, mammary gland hyperplasia, mammary gland fibroadenoma and (in mice only) mammary gland adenocarcinoma. These effects occurred at plasma tamsulosin concentrations (AUC) up to 10 times lower than those expected in men undergoing treatment with tamsulosin, but they were observed only in female animals and are probably due to the hyperprolactinaemic effect of tamsulosin. It is not known if tamsulosin elevates prolactin during modified administration in humans. The relevance for human risk of the findings of prolactin-mediated endocrine tumours in female rodents is unknown.6 Pharmaceutical Particulars
6.1 List of Excipients
Tamsulosin-WGR SR 400 microgram modified release tablets contain the following inactive ingredients: microcrystalline cellulose, polyethylene oxide and magnesium stearate.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
6.4 Special Precautions for Storage
Tamsulosin-WGR SR 400 microgram modified release tablets should be stored below 25°C and protect from light.
6.5 Nature and Contents of Container
Tamsulosin-WGR SR is available in OPA/Al/PVC blister packs of 30 tablets.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Tamsulosin hydrochloride is sparingly soluble in water (1:85) and slightly soluble in alcohol. It is stable in an acid environment.
Chemical structure.
 Chemical name: 5-[(2R)-2-[2-(2-ethoxyphenoxy)ethylamino]propyl]-2-methoxybenzenesulfonamide hydrochloride.
Chemical name: 5-[(2R)-2-[2-(2-ethoxyphenoxy)ethylamino]propyl]-2-methoxybenzenesulfonamide hydrochloride.
Molecular formula: C20H29ClN2O5S.
Molecular weight: 445.0.
CAS number.
106463-17-6.7 Medicine Schedule (Poisons Standard)
S4 (Prescription Only Medicine).
Summary Table of Changes


 The following treatment-related adverse events were reported from clinical trials, where Common is ≥ 1% and < 10%; Uncommon is ≥ 0.1% and < 1%; Rare is ≥ 0.01% and < 0.1%; and Very rare is < 0.01%.
The following treatment-related adverse events were reported from clinical trials, where Common is ≥ 1% and < 10%; Uncommon is ≥ 0.1% and < 1%; Rare is ≥ 0.01% and < 0.1%; and Very rare is < 0.01%.

 As a result of the modified release characteristic of tamsulosin, the trough concentrations - at steady state, of tamsulosin hydrochloride in plasma amount to approximately 40% of the peak plasma concentrations, under fasted and fed conditions.
As a result of the modified release characteristic of tamsulosin, the trough concentrations - at steady state, of tamsulosin hydrochloride in plasma amount to approximately 40% of the peak plasma concentrations, under fasted and fed conditions. Chemical name: 5-[(2R)-2-[2-(2-ethoxyphenoxy)ethylamino]propyl]-2-methoxybenzenesulfonamide hydrochloride.
Chemical name: 5-[(2R)-2-[2-(2-ethoxyphenoxy)ethylamino]propyl]-2-methoxybenzenesulfonamide hydrochloride.