What is in this leaflet
This leaflet answers some common questions about TARGIN tablets.
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What TARGIN tablets are taken for
TARGIN tablets contain two different medicines called oxycodone hydrochloride and naloxone hydrochloride anhydrous. Oxycodone belongs to a group of medicines called opioid analgesics. Naloxone is a medicine which, when taken orally, can block some of the effects of opioids in the gut, such as constipation.
Pain relief:
TARGIN tablets are used to relieve severe pain when other forms of treatment have failed or are otherwise inappropriate to provide sufficient management of pain. The naloxone in TARGIN tablets will help prevent and treat opioid-induced constipation.
Restless legs syndrome:
TARGIN tablets are also used to relieve the symptoms of severe to very severe restless legs syndrome in people who can't be treated with dopamine medicines. People with restless legs syndrome have unpleasant sensations in their limbs. This can start as soon as they sit or lie down and is only relieved by an irresistible urge to move the legs, sometimes the arms and other parts of the body. It makes sitting still and sleeping very difficult.
TARGIN tablets helps to relieve the unpleasant sensations and so reduces the urge to move the limbs.
Your doctor, however, may prescribe it for another purpose.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
As with all strong painkillers, your body may become used to you taking TARGIN tablets. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking TARGIN tablets suddenly, so it is important to take it exactly as directed by your doctor.
This medicine is only available with a doctor's prescription.
Before you take TARGIN
Long-term use of TARGIN tablets may affect your fertility. If you have any concerns about taking this medicine, ask your doctor.
When you must not take it
Do not take TARGIN tablets if you:
- have moderate or severe liver disease
- have any breathing problems such as severe asthma, respiratory depression (breathing slows or weakens) or other obstructive airways disease
- are severely drowsy or have a reduced level of consciousness
- have a condition where your stomach empties more slowly than it should or any condition that obstructs the stomach/bowel or affects bowel transit (movement of food or ingested material along the bowel)
- have sudden severe abdominal pain
- have irregular heart beats or changes in the way the heart beats
- have a head injury, brain tumour or have raised pressure within the head, brain or spinal cord
- suffer from uncontrolled convulsions, fits or seizures
- have heart problems due to long-term lung disease
- have just consumed and/or regularly consume large amounts of alcohol or have confusion and shaking due to alcohol withdrawal
- take a medicine for depression called a 'monoamine oxidase inhibitor' or have taken any in the last two weeks
- are about to have an operation (including surgery on your spine for pain relief in the next 24 hours) or have had an operation within the last 24 hours
- have a history of opioid abuse.
Do not take TARGIN tablets if you are allergic to oxycodone, naloxone, opioid painkillers, or any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take this medicine after the expiry date (EXP) printed on the pack.
If you take it after the expiry date has passed, it may not work very well.
Do not take it if the packaging is torn or shows signs of tampering.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any medical conditions, especially the following:
- chronic kidney, liver or lung disease
- high or low blood pressure
- problems with your heart and blood circulation
- underactive thyroid gland
- disease of your gall bladder or bile duct
- inflammation of the pancreas
- inflammatory bowel disease or recent abdominal surgery
- enlarged prostate
- underactive adrenal glands
- epileptic disorders or are prone to having convulsions
- galactose intolerance, lactose deficiency or glucose-galactose malabsorption
- some cancers of the digestive tract or pelvis
- severe mental condition involving losing contact with reality, hearing voices or an ability to think clearly
- an addiction or history of abuse of alcohol, opioids or other drugs
- sleep apnoea, a condition characterised by frequent breathing stops during the night which may make you feel very sleepy during the daytime
- episodes of suddenly falling asleep or sleepiness occur.
Tell your doctor if you have used an opioid before. Using TARGIN tablets after using doses of other opioids may lead to withdrawal symptoms or diarrhoea.
If you have not told your doctor about any of the above, tell them before you take any TARGIN tablets.
Pregnancy and breastfeeding
Do not take this medicine if you are pregnant or intend to become pregnant whilst taking this medicine.
Like most medicines of this kind, TARGIN tablets are not recommended to be taken during pregnancy. Your doctor will discuss the risks of taking it if you are pregnant.
Do not breastfeed if you are taking this medicine.
The active ingredients in TARGIN tablets may pass into breast milk and there is a possibility that your baby may be affected. Your doctor can discuss with you the risks involved.
Do not give this medicine to a child younger than 18 years of age.
Safety and effectiveness in children younger than 18 years of age have not been established.
Addiction
You can become addicted to TARGIN even if you take it exactly as prescribed. TARGIN may become habit forming causing mental and physical dependence. If abused it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking TARGIN. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking TARGIN suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to TARGIN may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue taking your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
Taking other medicines
Tell your doctor if you are taking any other medicines or dietary supplements, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines, alcohol and TARGIN tablets may interfere with each other. These medicines include:
- medicines used to treat depression, psychiatric or mental disorders
- medicines used to treat depression belonging to a group called monoamine oxidase inhibitors must be stopped 14 days before TARGIN tablets are taken
- antidepressants e.g. citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, venlafaxine.
- medicines to help you sleep
- other pain relievers including other opioids
- medicines used to prevent or relieve the symptoms of allergy
- medicines to stop nausea or vomiting e.g. metoclopramide or prochlorperazine
- cimetidine, a medicine used to treat stomach ulcers or heartburn
- medicines to put you to sleep during an operation or procedure
- medicines used to thin the blood e.g. coumarin derivatives such as warfarin
- medicines used to lower blood pressure
- quinidine and other medicines used to treat the heart
- medicines to treat convulsions e.g. phenytoin, carbamazepine
- antifungals e.g. ketoconazole
- antibiotics e.g. clarithromycin, rifampicin
- medicines to treat HIV infection and AIDS e.g. ritonavir
- medicines used to relieve stomach cramps or spasms, to prevent travel sickness
- medicines to treat Parkinson's disease
- medicines to treat urinary incontinence
- medicines used to relax muscles
- St John's wort (a herbal preparation)
- grapefruit and grapefruit juice.
- medicines to treat epilepsy, pain, and anxiety e.g. gabapentin and pregabalin.
These medicines, dietary supplements or alcohol may affect how well TARGIN tablets work, or may increase side effects. You may need to avoid these or use different amounts, or take different medicines.
While you are taking this medicine for restless legs syndrome your doctor may reduce your dose if you are taking TARGIN tablets and Rotigotine, a medicine used to treat Parkinson's disease.
Your doctor or pharmacist has more information on medicines and dietary supplements to be careful with or avoid while taking this medicine.
How to take TARGIN tablets
How much to take
Your doctor will tell you exactly how much to take.
Follow the instructions given to you by your doctor or pharmacist exactly.
How to take it
Swallow TARGIN tablets whole with a full glass of water.
Do not chew, crush, break or dissolve the tablets. TARGIN tablets are only designed to work properly if swallowed whole. The tablets may release all their contents at once if chewed, crushed, broken or dissolved which can be dangerous and cause serious problems, such as an overdose which may be fatal.
If you have trouble swallowing your tablets whole, talk to your doctor.
You must only take TARGIN tablets by mouth. Taking this medicine in a manner other than that prescribed by your doctor can be harmful to your health.
When to take it
Take TARGIN tablets every 12 hours.
Take TARGIN tablets regularly to control the pain or to relieve the symptoms of restless legs syndrome.
Taking them at the same time each day will assist in ensuring the best effect in improving your pain. If however, you begin to experience worsening pain or symptoms and you are taking your TARGIN tablets as prescribed, contact your doctor as your dosage may need to be reviewed.
You may take this medicine with or without food.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
If you stop taking this medicine suddenly, your pain or symptoms may worsen and you may experience withdrawal symptoms such as:
- body aches
- loss of appetite, nausea, stomach pain or diarrhoea
- fast heart rate
- sneezing or runny nose
- chills, tremors, shivering or fever
- trouble with sleeping
- increased sweating and yawning
- weakness
- nervousness or restlessness.
If you forget to take it
If you forget to take your tablets, contact your doctor or pharmacist for advice.
Do not take a double dose to make up for the dose that you missed. This will increase the chance of you getting unwanted side effects.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your tablets, ask your pharmacist for some hints. For example, take your medicine at the same time each morning and evening such as 8 a.m. and 8 p.m.
If you take too much (overdose)
If you or someone else receive too much (overdose), and experience one or more of the symptoms below, call triple zero (000) for an ambulance. Keep the person awake by talking to them or gently shaking them every now and then. You should follow the steps even if someone other than you have accidentally used TARGIN in that was prescribed for you. If someone takes an overdose, they may experience one or more of the following symptoms:
- slow, unusual or difficult breathing
- drowsiness, dizziness or unconsciousness
- slow or weak heartbeat
- nausea or vomiting
- convulsions or fits
If you think you or someone else may have taken too much TARGIN, you should immediately telephone your doctor or the Poisons Information Centre (Australia: telephone 13 11 26) , or go to Emergency Department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning.
349366-16
While you are taking TARGIN
Things you must do
Take TARGIN tablets exactly as your doctor has prescribed.
Before you start on a new medicine, remind your doctor and pharmacist that you are taking TARGIN tablets.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor's appointments so that your progress can be checked.
Tell your doctor if your pain is getting worse, or if you are having more frequent breakthrough pain.
Also tell your doctor if you are having any problems or difficulties while you are being treated with TARGIN tablets.
Tolerance to oxycodone may develop which means that the effect of the medicine may decrease. If this happens, your doctor may review your dose so that you get adequate pain relief.
Keep enough TARGIN tablets with you to last over weekends and holidays.
Things you must not do
Do not drink alcohol while you are taking TARGIN tablets. Drinking alcohol while taking TARGIN tablets may make you feel more sleepy and increase the risk of serious side effects, such as shallow breathing with the risk of stopping breathing and loss of consciousness.
Do not take TARGIN tablets to treat any other complaint unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or change the dosage without checking with your doctor.
Over time your body may become used to you taking oxycodone so if you stop taking it suddenly, your pain may worsen and you may have unwanted side effects such as withdrawal symptoms. This is called physical dependence.
If you need to stop taking this medicine, your doctor will gradually reduce the amount you take each day, if possible, before stopping the medicine completely.
Things to be careful of
Do not drive or operate machinery until you know how TARGIN tablets affect you. TARGIN tablets may cause episodes of suddenly falling asleep, drowsiness, dizziness, hallucinations, disorientation, blurred vision or other vision problems or may affect alertness. If you are affected, you should not drive or operate machinery. Discuss these effects with your doctor.
If you have been switched to TARGIN tablets from other opioid pain medications and are taking laxatives, you may need to reassess your laxative treatment. It may be appropriate to reduce your laxative intake when you start taking TARGIN tablets and throughout your TARGIN tablet therapy.
Be careful if you are elderly, unwell or taking other medicines. Some people may experience side effects such as drowsiness, confusion, dizziness and unsteadiness which may increase the risk of a fall.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly. Standing up slowly will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Tell your doctor if you suffer from nausea or vomiting when taking TARGIN tablets. If you vomit after your dose, your pain may come back, as you will not have absorbed your medicine. If this happens, speak to your doctor. Your doctor may prescribe some medicine to help you stop vomiting.
Do not be alarmed if you see remnants of the tablet in your stool. The active substances have already been released in the stomach and gut, and absorbed into your body.
There is potential for abuse of oxycodone and the development of addiction to oxycodone. It is important that you discuss this issue with your doctor.
Be aware that TARGIN tablets may produce a positive result in sports agency drug testing.
Side effects
All medicines may have some unwanted side effects. Sometimes they are serious, but most of the time they are not. As for other medicines of this type, that is opioid analgesics, many side effects tend to reduce over time. This means that the longer you take this medicine, the less it may cause problems for you. Your doctor has weighed the risks of this medicine against the benefits they expect it will have for you.
Do not be alarmed by this list of possible side effects. Not everybody experiences them.
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking TARGIN tablets.
This medicine helps most people with moderate to severe pain, but it may have unwanted side effects in a few people. Other side effects not listed here may also occur in some people.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- mild abdominal problems such as diarrhoea, feeling sick (nausea), decreased appetite, constipation
- dry mouth, hiccups, sore throat, thirst, trouble swallowing or changes in voice
- feeling anxious or nervous, trouble sleeping or abnormal dreams
- trouble with your balance
- excessive sweating, hot flushes
- restlessness
- muscle problems such as spasms, twitching or tremors
- fatigue and feeling of tiredness
- skin rash, itching, chills, or fever
- new problems with your eyesight
- swelling of legs and ankles
- absence of menstrual period
- impotence
- decreased sexual drive.
Tell your doctor as soon as possible if you notice any of the following and they worry you:
- stomach discomfort, vomiting, indigestion or abdominal pain
- abnormal thinking or changes in mood, or feeling deep sadness
- slow or noticeable heart beats
- headache or confusion
- drowsiness, feeling faint or fainting or dizziness especially when standing up
- unusual weakness, loss of strength or trouble walking
- urinary tract infections or change in passing urine such as the volume passed, pain or feeling the need to urinate urgently.
The above list includes serious side effects that may require medical attention.
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- you have an allergic reaction: shortness of breath, wheezing, shallow or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body; rash, itching or hives on the skin
- your breathing slows or weakens
- chest pain or chest tightness
- fast or irregular heartbeats
- seizures, fits or convulsions.
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
After taking TARGIN
Storage
Keep your tablets in the blister pack until it is time to take them. If you take the tablets out of the blister pack they may not keep as well.
Keep your tablets in a cool, dry place where the temperature stays below 25°C.
Do not store it or any other medicine in the bathroom, near a sink or on a window sill.
Do not leave it in the car. Heat and damp can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
Product description
What it looks like
TARGIN® tablets are modified release film-coated tablets. They are available in seven strengths which are as follows*:
2.5/1.25 mg - round, light yellow with no markings
5/2.5 mg - oblong, blue, marked "OXN" on one side and "5" on the other
10/5 mg - oblong, white, marked "OXN" on one side and "10" on the other
15/7.5 mg - oblong, grey, marked "OXN" on one side and "15" on the other
20/10 mg - oblong, pink, marked "OXN" on one side and "20" on the other
30/15 mg - oblong, brown, marked "OXN" on one side and "30" on the other
40/20 mg - oblong, yellow, marked "OXN" on one side and "40" on the other.
60/30 mg - oblong, red, marked "OXN" on one side and "60" on the other
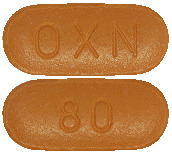
80/40 mg - oblong, brown, marked "OXN" on one side and "80" on the other
*Not all strengths are marketed in Australia
TARGIN® tablets come in boxes containing blister packs of 28 tablets.
Ingredients
Active ingredients:
- 2.5/1.25 mg contains 2.5 mg oxycodone hydrochloride and 1.25 mg naloxone hydrochloride
- 5/2.5 mg contains 5 mg oxycodone hydrochloride and 2.5 mg naloxone hydrochloride
- 10/5 mg contains 10 mg oxycodone hydrochloride and 5 mg naloxone hydrochloride
- 15/7.5 mg contains 15 mg oxycodone hydrochloride and 7.5 mg naloxone hydrochloride
- 20/10 mg contains 20 mg oxycodone hydrochloride and 10 mg naloxone hydrochloride
- 30/15 mg contains 30 mg oxycodone hydrochloride and 15 mg naloxone hydrochloride
- 40/20 mg contains 40 mg oxycodone hydrochloride and 20 mg naloxone hydrochloride.
- 60/30 mg contains 60 mg oxycodone hydrochloride and 30 mg naloxone hydrochloride.
- 80/40 mg contains 80 mg oxycodone hydrochloride and 40 mg naloxone hydrochloride.
Inactive ingredients:
- lactose
- hyprolose (2.5/1.25mg, 5/2.5mg and 15/7.5mg tablets)
- povidone (10/5mg, 20/10mg, 30/15mg, 40/20mg, 60/30 mg and 80/40 mg tablets)
- ethylcellulose
- stearyl alcohol
- purified talc
- magnesium stearate
- polyvinyl alcohol
- titanium dioxide
- macrogol 3350
- brilliant blue FCF (CI42090) (5/2.5mg tablets)
- iron oxide red (CI77491) (2.5/1.25mg, 15/7.5mg, 20/10mg 30/15mg and 60/30 mg tablets)
- iron oxide yellow (CI77492) (2.5/1.25mg, 15/7.5mg, 30/15mg 40/20mg and 80/40 mg tablets)
- iron oxide black (CI77499) (15/7.5mg, 30/15mg, 60/30 mg and 80/40 mg tablets).
This medicine does not contain sucrose, gluten, tartrazine or any other azo dyes.
Supplier
TARGIN® tablets are supplied in Australia by:
Mundipharma Pty Limited
ABN 87 081 322 509
10 Carrington Street
Sydney NSW 2000
Phone: 1800 188 009
® TARGIN is a trade mark of MUNDIPHARMA.
This leaflet was updated in June 2023
Please check with your pharmacist that this is the latest version of the leaflet available.
Australian Registration Numbers for TARGIN® tablets are:
2.5/1.25 mg AUST R 216260
5/2.5 mg AUST R 156067
10/5 mg AUST R 156145
15/7.5 mg AUST R 216261
20/10 mg AUST R 156189
30/15 mg AUST R 216280
40/20 mg AUST R 156194
60/30 mg AUST R 243252
80/40 mg AUST R 243272
targin-CMIv2-CCDSv15
Published by MIMS September 2023









 It is emphasised that this is a guide to the required dose of Targin modified release tablets only. Inter-patient variability requires that each patient is carefully titrated to the appropriate dose. The correct dosage for any individual patient is the minimum dose that controls the pain, provides functional improvement and is well tolerated, for a full 12 hours. Patients should be titrated to pain relief and functional improvement unless unmanageable adverse drug reactions prevent this.
It is emphasised that this is a guide to the required dose of Targin modified release tablets only. Inter-patient variability requires that each patient is carefully titrated to the appropriate dose. The correct dosage for any individual patient is the minimum dose that controls the pain, provides functional improvement and is well tolerated, for a full 12 hours. Patients should be titrated to pain relief and functional improvement unless unmanageable adverse drug reactions prevent this.

 Adverse drug reactions attributable to Targin modified release tablets were reported at the frequencies below:
Adverse drug reactions attributable to Targin modified release tablets were reported at the frequencies below:


 The results show a clinically relevant and statistically significant improvement of the BFI scores in the Targin group compared to oxycodone CR tablets. The improvements consistently appeared in the Full Analysis (FA) as well as the Per Protocol (PP) population, in all the subgroups, in sensitivity analysis with Last Observation Carried Forward (LOCF) imputation, and in all 3 single BFI parameters. See Table 9.
The results show a clinically relevant and statistically significant improvement of the BFI scores in the Targin group compared to oxycodone CR tablets. The improvements consistently appeared in the Full Analysis (FA) as well as the Per Protocol (PP) population, in all the subgroups, in sensitivity analysis with Last Observation Carried Forward (LOCF) imputation, and in all 3 single BFI parameters. See Table 9. In the FA population at week 1 the mean BFI decreased by -28.3 in the Targin group and by -13.1 in the oxycodone CR tablets group. A median decrease of -23.3 in the Targin group compared with -6.7 in the oxycodone CR tablets group was observed.
In the FA population at week 1 the mean BFI decreased by -28.3 in the Targin group and by -13.1 in the oxycodone CR tablets group. A median decrease of -23.3 in the Targin group compared with -6.7 in the oxycodone CR tablets group was observed.
 The structural formula for naloxone hydrochloride dihydrate is:
The structural formula for naloxone hydrochloride dihydrate is:
