What is in this leaflet
This leaflet is designed to provide you with answers to some common questions about this medicine. It does not contain all the available information that is known about Tazac.
It does not take the place of talking with your doctor.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Tazac against the benefits they expect it will have for you.
If you have any concerns about taking this medicine ask your doctor or pharmacist.
Keep this leaflet with this medicine. You may need to read it again.
What TAZAC is used for
Tazac contains an active ingredient called nizatidine. It belongs to a class of medicines called H2-antagonists or H2-blockers.
Tazac is used to treat the following conditions:
Reflux oesophagitis
This can be caused by reflux or "washing back" of food and acid from the stomach into the food pipe. Reflux can cause a burning sensation in the chest rising up to the throat, also known as heartburn.
Ulcers
Depending on the position of the ulcer it is either called a gastric or duodenal ulcer.
A gastric ulcer occurs in the stomach. A duodenal ulcer occurs in the duodenum which is the tube leading out of the stomach. Tazac is also used to stop duodenal ulcers from coming back.
Tazac works by reducing the amount of acid in your stomach. This helps reduce the pain and allows the ulcer and reflux disease to heal in most people.
Ask your doctor or pharmacist if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is only available with a doctor's prescription.
There is no evidence that it is addictive.
Before you take it
When you must not take it
Do not take Tazac if you have ever had an allergic reaction to:
- nizatidine or other histamine H2-receptor antagonists (e.g. cimetidine, ranitidine, famotidine)
- any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty in breathing; swelling of the face, lips, tongue or any other parts of the body; rash, itching or hives on the skin.
Do not take Tazac after the expiry printed on the pack. If you take this medicine after the expiry date has passed it may not work as well.
Do not take Tazac if the packaging is torn or shows signs of tampering.
Before you start to take it
Tell your doctor if you are allergic to any other medicines or any foods, dyes or preservatives.
Tell your doctor if you:
- are pregnant or intend to become pregnant
- are breast feeding or plan to breast feed
- have kidney or liver disease.
If you have not told your doctor about any of these things, tell them before you take Tazac.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may affect the way other medicines work. Medicines such as ketoconazole and itraconazole used to treat fungal infections may be affected by Tazac. Your doctor or pharmacist will be able to tell you what to do when taking Tazac with other medicines.
How to take it
How much to take
Depending on your condition, your doctor will tell you how much Tazac to take each day.
When to take it
The 150 mg capsule is usually taken in the morning and in the evening before you go to bed.
The 300 mg capsule is usually taken once daily, at bedtime.
How to take it
Swallow the capsule whole with a glass of water or another liquid.
How long do I take it
Do not stop taking the capsules just because you feel better.
Your doctor will tell you how long you should continue taking Tazac. If you stop taking your capsules too early then your condition may not have been properly treated.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to taking Tazac as you would normally.
If you are not sure whether to skip the dose, talk to your doctor or pharmacist.
Do not take a double dose to make up for the dose that you missed.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital if you think that you or anyone else may have taken too much Tazac. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking it
Things you must do
Tell your doctor or pharmacist if you start any new medicine while you are taking Tazac.
If you are taking it for an ulcer, you should go to your doctor regularly for checkups to make sure that Tazac has healed your ulcer.
Tell your doctor immediately if you become pregnant while taking Tazac.
Things you must not do
Do not give this medicine to anyone else even if their symptoms seem similar to yours. Your doctor has prescribed it for you and your condition.
Do not take Tazac to treat any other complaints unless your doctor tells you to.
Things to be careful of
Be careful driving or operating machinery until you know how Tazac affects you. This medicine may cause dizziness or lightheadedness in some people. Make sure you know how you react to it before you drive a car or operate any machinery.
Your doctor may advise you to limit your alcohol intake while you are being treated for your condition.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Tazac.
Tazac helps most people who take it but it may have unwanted side effects in some people. All medicines have side effects. Sometimes they are serious, most of the time they are not. You may need medical treatment if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following common side effects and they worry you:
- sweating
- itchy skin or rash
- tiredness, headaches, being short of breath when exercising, dizziness and looking pale.
Incidences of abnormal liver function, accompanied by jaundice (yellow skin) have been rarely reported by patients taking this medicine. This side effect has been reversed when Tazac is stopped.
Tell your doctor if you notice anything unusual or if you are concerned about any aspect of your health, even if you think the problems are not connected with this medicine and are not referred to in this leaflet.
After taking it
Storage
Keep your capsules in the blister pack until it is time to take them. If you take your capsules out of the blister pack, they may not keep as well.
Keep your capsules in a cool dry place where the temperature stays below 25 degrees Celsius.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half metres above ground is a good place to store medicines.
Disposal
Dispose of the medicine where children cannot reach it.
If your doctor tells you to stop taking Tazac or you find that the expiry date has passed, ask your pharmacist what to do with any capsules you have left over.
Product Description
What it looks like
150 mg: Light and dark yellow capsule printed with "N150". Packs of 60 capsules.
300 mg: Light yellow and brown capsule printed with "N300". Packs of 30 capsules.
Ingredients
Tazac capsules contain 150 mg or 300 mg of nizatidine as the active ingredient.
The 150 mg capsule also contains the following inactive ingredients: maize starch, pregelatinised maize starch, dimeticone 350, magnesium stearate, iron oxide yellow, titanium dioxide, sodium lauryl sulfate, gelatin and printing Ink OPACODE monogramming ink S-1-17823 BLACK (PI 12108).
The 300 mg capsule also contains the following inactive ingredients: maize starch, pregelatinised maize starch, povidone, croscarmellose sodium, dimeticone 350, purified talc , iron oxide red, iron oxide yellow, titanium dioxide, gelatin and printing Ink Tekprint SW-09008 black ink (PI 2328) or printing Ink ACG PAM BKI004 (PI 139674)
Tazac does not contain gluten, lactose, sucrose, tartrazine or any other azo dyes. May contain traces of sulfites.
Distributor
Arrow Pharma Pty Ltd
15-17 Chapel Street
Cremorne VIC 3121
Australian Registration Numbers:
TAZAC 150 mg - AUST R 284130
TAZAC 300 mg - AUST R 49326
This leaflet was prepared in March 2024.
Published by MIMS April 2024


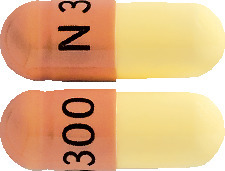
 Some elderly patients may have creatinine clearances of less than 50 mL/min, and, based on pharmacokinetic data in patients with renal impairment, the dose for such patients should be reduced accordingly. The clinical effects of this dosage reduction in patients with renal failure have not been evaluated.
Some elderly patients may have creatinine clearances of less than 50 mL/min, and, based on pharmacokinetic data in patients with renal impairment, the dose for such patients should be reduced accordingly. The clinical effects of this dosage reduction in patients with renal failure have not been evaluated.
