What is in this leaflet
Read this leaflet carefully before taking your medicine.
This leaflet answers some common questions about ranitidine. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is Terry White Chemists Ranitidine tablets. It contains the active ingredient ranitidine (as hydrochloride).
It is used to treat:
- Duodenal ulcer
- Gastric ulcer
- Reflux oesophagitis
- Scleroderma oesophagitis
- Zollinger-Ellison syndrome
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed this medicine for another reason.
This medicine is available only with a doctor's prescription.
How it works
Ranitidine belongs to a group of medicines called H2 antagonists or H2 blockers.
Ranitidine works by decreasing the amount of acid made by the stomach. This helps reduce pain and also allows an ulcer and/or reflux disease to heal in most people.
There is no evidence that this medicine is addictive.
Use in children
Experience with ranitidine preparations in children is limited and such use has not been fully evaluated in clinical studies. It has, however, been used successfully in children aged 8 to 18 years in doses up to 150 mg twice daily.
Before you take this medicine
When you must not take it
Do not take this medicine if:
- You are hypersensitive to, or have had an allergic reaction to, ranitidine or any of the ingredients listed at the end of this leaflet.
- The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You are allergic to foods, dyes, preservatives or any other medicines
- You have ever had an allergic reaction to ranitidine or any of the ingredients listed at the end of this leaflet
- You are allergic to any medicine
- You have had stomach ulcers before and you are taking non-steroidal anti-inflammatory (NSAID) medicines
- You have kidney disease
- You have liver disease
- You have a disease known as porphyria (rare disease of blood pigments)
- You have lung disease, cough or a chest infection
- You are diabetic
- You have problems with your immune system
- You have stomach cancer
- You are over 65 years of age.
- You are pregnant, likely to get pregnant or are breastfeeding. Your doctor will tell you if you should take this medicine.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, have taken any recently or if you start any new ones. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop with or without a prescription.
Some medicines may interact with ranitidine. These include:
- sucralfate, another medicine used to treat peptic ulcer
- ketoconazole, a medicine used to treat fungal infection
- heart medication e.g. procainamide
- anticoagulant e.g. warfarin
- benzodiazepines e.g. triazolam, midazolam
- antiretrovirals e.g. atazanavir, delavirdine
- antidiabetic e.g. glipizide
- anticancer e.g. gefitinib
- Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), used to treat pain and inflammation.
If you are taking any of the above medicines, you may need a different dose or you may need to take different medicines.
Other medicines not listed above may also interact with ranitidine.
How to take this medicine
Follow carefully all directions given to you by your doctor. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor will tell you how much of this medicine you should take. This will depend on your condition and whether you are taking any other medicines.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
How to take it
Swallow each tablet whole with a glass of water.
When to take it
Take this medicine at the same time each day. Taking it at the same time each day will have the best effect and will also help you remember when to take it.
It does not matter if you take it before, with or after food.
If ranitidine is prescribed twice a day, the best time to take it is in the morning and at bedtime.
If ranitidine is prescribed once daily, the best time to take it is at bedtime.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How long to take it
Continue taking your medicine for as long as your doctor tells you.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is almost time to take your next dose, skip the missed dose and take your next dose at the usual time. Otherwise, take it as soon as you remember and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for missed doses. This may increase the chance of you experiencing side effects.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively, go to the Accident and Emergency department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are taking this medicine
Things you must do
Tell your doctor that you are taking this medicine if:
- you are about to be started on any new medicine
- you are pregnant or are planning to become pregnant
- you are breastfeeding or are planning to breastfeed
- you are about to have any blood tests
- you are going to have surgery or an anaesthetic or are going into hospital.
Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects. Go to your doctor regularly for a check-up.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- Give this medicine to anyone else, even if their symptoms seem similar to yours.
- Take your medicine to treat any other condition unless your doctor tells you to.
- Stop taking your medicine, or change the dosage, without first checking with your doctor.
Things to be careful of
Be careful when driving or operating machinery until you know how this medicine affects you.
Ranitidine generally does not cause any problems with your ability to drive a car or operate machinery. However, as with many other medicines, ranitidine may cause dizziness or light-headedness in some people.
Side effects
Tell your doctor as soon as possible if you do not feel well while you are taking ranitidine or if you have any questions or concerns.
Do not be alarmed by the following lists of side effects. You may not experience any of them. All medicines can have side effects. Sometimes they are serious but most of the time they are not.
Tell your doctor if you notice any of the following:
- headache
- dizziness or light-headedness
- uncontrolled movements. This effect is reversible and should get better once you stop taking this medicine
- if you are a man, breast tenderness and/or breast enlargement; loss in sexual desire or performance. The interference with sexual function is normally reversible and should get better once you stop taking this medicine
- inflammation of blood vessels (vasculitis)
- abdominal discomfort or pain
- constipation or diarrhoea (especially if antibiotics are also being taken)
- sore joints and/or muscles
- feeling of agitation or depression
- hallucinations
- loss of hair.
Inform your doctor immediately if you get any signs of liver problems which may cause one or more of the following:
- generally feeling unwell
- severe nausea (feeling sick)
- loss of appetite
- development of yellowish tinge to skin and/or eyes (jaundice)
- fever
- rash and/or itching
- dark coloured urine
- confusion
- muscle tremor
- vomiting (being sick)
- severe stomach pain rarely caused by inflamed pancreas
- blurred vision.
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
- unusual tiredness, shortness of breath or tendency to infections or bruising, which can be caused by upsets to "blood counts"
- changes in the way the heart beats; either faster or slower
- severe stomach pain or a change in the type of pain
- symptoms of an allergic reaction including cough, shortness of breath, wheezing of difficulty breathing; swelling of the face, lips, tongue, throat; rash, itching or hives on the skin; fainting and/or hayfever-like symptoms.
Other problems are more likely to arise from the ulcer itself rather than the treatment. For this reason, contact your doctor immediately if you notice any of the following:
- pain or indigestion occurring during treatment with ranitidine
- you begin to vomit blood or food
- your pass black (blood-stained) motions.
Other side effects not listed above may occur in some patients.
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it.
If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C. Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What Terry White Chemists Ranitidine looks like
Terry White Chemists Ranitidine tablets are available in two different strengths:
- Terry White Chemists Ranitidine 150 mg tablets: white to off-white, round, biconvex film coated tablets. Scored on one side and engraved "RAN" over "150" on the other side.
Available in blister packs of 60.
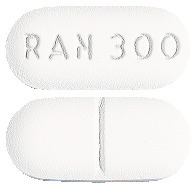
- Terry White Chemists Ranitidine 300 mg tablets: white to off-white, capsule-shaped, biconvex film coated tablets. Scored on one side and engraved "RAN 300" on the other side
Available in blister packs of 30.
* Not all strengths, pack types and/or pack sizes may be available.
Ingredients
Each Terry White Chemists Ranitidine tablets contains 150 mg of ranitidine (as ranitidine hydrochloride) as the active ingredient.
Each Terry White Chemists Ranitidine tablets contains 300 mg of ranitidine as (as ranitidine hydrochloride) the active ingredient.
Terry White Chemists Ranitidine tablets also contains the following inactive ingredients:
- Microcrystalline cellulose
- magnesium stearate
- colloidal anhydrous silica
- hypromellose
- polydextrose
- titanium dioxide
- vanillin
- carnauba wax.
Terry White Chemists Ranitidine tablets are gluten-free, lactose-free, sucrose-free, tartrazine-free and free of other azo dyes.
Australian Registration Numbers
Terry White Chemists Ranitidine 150 mg tablets blister pack: AUST R 121975.
Terry White Chemists Ranitidine 300 mg tablets blister pack: AUST R 121978.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
This leaflet was last updated in: March 2019.
Published by MIMS May 2019