What is in this leaflet
This leaflet answers some common questions about TINASIL tablets.
It does not contain all the available information about TINASIL tablets. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. Some more recent information on the medicine may be available.
You should ensure that you speak to your pharmacist or doctor to obtain the most up to date information on the medicine. Those updates may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will provide.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What TINASIL tablets are used for
TINASIL tablets are used to treat:
- fungal infections fingernails and toenails
- tinea (ringworm) infections of the groin and body
- tinea infections of the feet, commonly called "athlete's foot"
These infections are caused by a group of fungi called dermatophytes.
Terbinafine, the active ingredient in TINASIL tablets, works by killing the dermatophytes.
Ask your doctor if you have any questions about why this medicine has been prescribed for you. Your doctor may have prescribed it for another reason.
TINASIL tablets are only with a doctor's prescription.
This medicine is not addictive.
Do not use TINASIL in children.
Before you take TINASIL tablets
When you must not take it
Do not take TINASIL if you have ever had an allergic reaction to:
- terbinafine hydrochloride, the active ingredient, or to any of the other ingredients listed at the end of this leaflet
- any other medicines, foods, preservatives or dyes
Your doctor will want to know if you are prone to allergies.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Do not take TINASIL tablets:
- if you have any problems with your kidneys
- if you have or ever had a problem with your liver .
TINASIL is not recommended if you currently have a liver problem because it may make the problem worse. If you had a liver problem in the past and your liver is functioning normally now, your doctor may prescribe TINASIL tablets but may want to check your liver function before and during treatment with this medicine. Your doctor might take blood tests to monitor your liver function. In case of abnormal test results, he/she may ask you to stop taking TINASIL.
Do not take TINASIL tablets after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
In that case, return it to your pharmacist. Before you start to take it
Tell your doctor if you:
- are pregnant or plan to become pregnant
TINASIL is not recommended during pregnancy. If your doctor thinks it is necessary for you to take it, he/she will discuss with you the benefits and risks involved.
- are breast-feeding
Breastfeeding is not recommended since terbinafine, the active ingredient in TINASIL tablets, passes into breast milk. There is a possibility that your baby could be affected.
- have any skin problems such as rash, red skin, blistering of the lips, eyes or mouth, skin peeling, fever (possible signs of serious skin reactions), rash due to high level of a specific type of white blood cells (eosinophilia), serious skin reactions (e.g. Stevens-Johnson syndrome, a serious disorder of the skin with symptoms such as blisters, weakness, fever or toxic epidermal necrolysis)
- have any blood disorders
or experience weakness, unusual bleeding, bruising or frequent infections
- have or experience thickened patches of red/silver skin (psoriasis)
or facial rash, joint pain, muscle disorder, fever (cutaneous and systemic lupus erythematosus).
If you are not sure whether you should start taking TINASIL tablets, talk to your doctor or pharmacist.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and TINASIL tablets may interfere with each other. These include:
- some medicines used to treat depression and other mental disorders, including obsessive-compulsive disorders and panic attacks (e.g. some antidepressants such as tricyclic antidepressants, selective serotonin reuptake inhibitors including antiarrhythmics class 1A, 1B and 1C, monoamine oxidase inhibitors Type B, desipramine)
- some medicines for Parkinson's disease
- some medicines used to treat an irregular heartbeat, heart problems, high blood pressure and migraines (e.g. metoprolol)
- some medicines used to treat stomach ulcers (e.g. cimetidine)
- some medicines called antibiotics used to treat infectious diseases (e.g. rifampicin) caffeine
- ciclosporin, a medicine used to help prevent organ transplant rejection or to treat certain problems with the immune system.
- oral contraceptives (birth control pills). You may have problems, such as bleeding between periods, while you are taking TINASIL tablets
- warfarin, a medicine used to prevent blood clots
- Drugs used for treatment of cough e.g. dextromethorphan
- Other antifungal medicines such as Fluconazole, ketoconazole
You may need to take different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information.
If you have not told your doctor about any of these things, tell him/ her before you start taking this medicine.
How to take TINASIL tablets
Follow all directions given to you by your doctor and pharmacist carefully. These directions may differ from the information contained in this leaflet.
If you do not understand the instructions on the label, ask your doctor or pharmacist for help.
How much to take
Follow your doctor's instructions on how many TINASIL tablets to take.
The usual dose of TINASIL is one tablet (250 mg) each day. If you have kidney problems, the dose may be reduced to one-half a tablet each day.
How to take it
Swallow the tablet with a full glass of water.
If your doctor has advised that you take half a tablet, you may divide the tablet in half along the breakline.
If you find that TINASIL upsets your stomach, try taking it immediately after a light meal.
Taking your tablet at the same time each day will have the best effect. It will also help you remember when to take it.
How long to take it
The length of your treatment will depend on the type of infection you have, what part of the body is affected and how well you respond to treatment. Your doctor will advise you regarding the duration of treatment.
Fungal skin infections (tinea):
If you have a tinea infection of the feet (Athlete's foot), you will usually take TINASIL tablets for 2 to 6 weeks.
If you have a tinea infection of the body or groin, you will usually take the tablets for 2 to 4 weeks.
The signs and symptoms of infection may last for several weeks after the fungi (dermatophytes) have been killed.
Fungal nail infections:
Fungal nail infections usually take longer to heal than fungal skin infections. You will usually take the tablets for anywhere from 6 weeks to 3 months. But, if you have a nail infection of the big toe or your nails grow very slowly, you may need to take the tablets for up to 6 months.
It may take several months after you stop taking TINASIL for your nail to look completely normal. That is because the deformed part of the nail has to grow out and be replaced by a healthy nail.
If your fungal infection does not improve. Your doctor will review and determine if an alternative antifungal therapy is needed.
If you forget to take it
If it is almost time for your next dose (within 4 hours), skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (Overdose)
Immediately telephone your doctor or Poisons Information Centre (telephone number 13 11 26), or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much TINASIL tablets. Do this even if there are no signs of discomfort or poisoning. Keep the telephone numbers for these places handy.
Some of the symptoms of an overdose may include headache, nausea (feeling sick), stomach pain and dizziness.
While you are taking TINASIL tablets
Things you must do
Make sure to take your tablet every day and continue taking it until your doctor tells you to stop. This will ensure that all of the infection is gone and will lessen the chance of the infection coming back once you stop taking the tablets.
Make sure to have any blood tests done that are ordered by your doctor.
Any effects of TINASIL on your liver, kidneys or blood can be detected by blood tests.
Tell your doctor immediately if you notice any of the following:
- fever
- sore throat
- mouth ulcers
- "flu-like" symptoms (chills, aching joints, swollen glands, lack of energy)
- any other signs of infection, apart from the fungal infection you are being treated for
If you become pregnant while taking TINASIL, tell your doctor immediately. Your doctor can discuss with you the risks and benefits of taking it during pregnancy.
Remind your doctor and pharmacist that you are about to be started on any new medicine. Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
Things you must not do
Do not give this medicine to anyone else, even if their condition seems similar to yours.
Do not take TINASIL tablets to treat any other complaints unless your doctor tells you to.
Things to be careful of
Be careful driving, operating machinery or doing jobs that require you to be alert while you are taking TINASIL tablets until you know how it affects you. This medicine can cause tiredness, sleepiness, dizziness or light-headedness in some people. If you have any of these symptoms, do not drive, use machines, or do anything else that could be dangerous.
Be careful to keep the infected areas dry and cool and change clothing that is in direct contact with the infected areas every day. This will help to clear up the infection and make sure that it does not return.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking TINASIL, even if you do not think it is connected with the medicine.
All medicines can have side effects. Sometimes they are serious, but most of the time they are not. You may need medical treatment if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- chest pain
- signs of a severe allergic reaction such as swelling of the face, lips, tongue, throat or other part of the body; shortness of breath, wheezing or troubled breathing; dizziness, redness, itching or rash on the skin; flushing, crampy abdominal pain, loss of consciousness, joint pain, stiffness, rash, fever or swollen/ enlarged lymph nodes
- possible signs of a serious liver problem such as persistent nausea, loss of appetite, unusual tiredness, vomiting, pain in the upper right abdomen, yellowing of the skin and/or eyes, dark urine or pale bowel motions, blood inthe stools (swelling of the stomach lining)
- possible signs of a serious skin reaction such as painful red areas, large blisters, peeling of layers of skin, bleeding in the lips, eyes, mouth, nose or genitals. These signs may be accompanied by fever and chills, aching muscles and feeling generally unwell
- possible signs of a blood problem such as constant "flu-like" symptoms (fever, sore throat, mouth ulcers, chills, swollen glands, lack of energy, chest pain, muscle stiffness)
- possible signs of diseases that affect certain types of blood cells: unusual bleeding or bruising
- possible signs of a disease that affects the level of red blood cells including abnormal pale skin, mucosal lining or nail beds, unusual tiredness or weakness or breathlessness on exertion
- possible signs of blood vessel inflammation: rash, fever, weight loss, itching, tiredness or if you notice appearance of purplish-red spots under the skin surface
- possible signs of pancreas inflammation: severe upper stomach pain with radiation to the back
- possible signs of muscle necrosis: unexplained muscle weakness and pain or dark (red-brown) urine.
- Other problems including hearing problems, partial loss of hearing, impaired hearing and/or perception of noises, taste disturbance, loss of taste, decreased ability to smell or detect odours, loss of the sense of smell
The above are serious side effects that need medical attention. Serious side effects are rare.
Tell your doctor if you notice any of the following and they worry you:
- nausea (feeling sick) or vomiting
- upset stomach (heartburn, cramps, wind, belching), indigestion, excessive burping, swelling of your belly
- loss of appetite
- diarrhoea
- aching joints or muscles
- headache
- dizziness or light headedness
- tiredness, sleepiness
- skin rash due to high level of a specific type of white blood cells
- loss of or change in sense of taste, which usually returns to normal within several weeks of stopping TINASIL
- blurred vision, decreased sharpness of vision
- other skin problems
- psoriasis (thickened patches of red skin, often with silvery scales)
- hair loss
- tingling or numbness
- decreased physical sensitivity
- anxiety (with symptoms such as sleep disturbances, fatigue, loss of energy or diminished ability to think or concentrate) and depressive symptoms (e.g. depressed mood) due to taste disturbances
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Some people may have other side effects not yet known or mentioned in this leaflet.
After using TINASIL tablets
Storage
Keep your tablets in the original container until it is time to take them.
Keep your tablets in a cool dry place where the temperature stays below 25°C.
Do not store TINASIL tablets or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
TINASIL tablets are round, white to off-white tablets uncoated, scored, biconvex tablets with "TF" over a breakline over "250" on one side and "G" on the other side. TINASIL tablets are supplied in blister packs containing 42 tablets..
Ingredients
TINASIL tablets contain 250 mg of the active ingredient, terbinafine (as the hydrochloride salt). They also contain the following inactive ingredients:
- microcrystalline cellulose
- croscarmellose sodium
- povidone
- colloidal anhydrous silica-
- purified talc
- magnesium stearate.
Supplier
TINASIL is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian Registration Number.
TINASIL 250 mg tablet
Blister packs - AUST R 104493
TINASIL® is a Viatris company trademark
This leaflet was revised in
October 2025.
TINASIL_cmi\Oct25/00
Published by MIMS December 2025

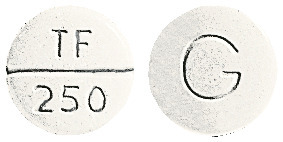
 Chemical name: (2E)-N,6,6-trimethyl-N- (naphthalene-1-ylmethyl)hept-2-en- 4-yn-1-amine hydrochloride.
Chemical name: (2E)-N,6,6-trimethyl-N- (naphthalene-1-ylmethyl)hept-2-en- 4-yn-1-amine hydrochloride.