Notes
Distributed by Generic Health Pty Ltd
1 Name of Medicine
Tiotropium (as tiotropium bromide monohydrate).
2 Qualitative and Quantitative Composition
Tiotropium Lupin is a generic version of Spiriva. Each capsule of Tiotropium Lupin and Spiriva delivers 10 micrograms of tiotropium and are equivalent.
Each Tiotropium Lupin capsule contains 18 micrograms tiotropium, equivalent to 22.5 micrograms tiotropium bromide monohydrate.
The delivered dose (the dose that leaves the mouthpiece of the LupinHaler device) is 10 micrograms of tiotropium.
Excipients with known effect.
Lactose monohydrate.
For the full list of excipients, see Section 6.1 List of Excipients.3 Pharmaceutical Form
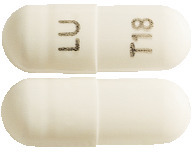
Tiotropium Lupin capsules are opaque white in colour, imprinted with "LU" on cap and "T18" on body with black ink. The capsules contain a white powder.
4.1 Therapeutic Indications
Tiotropium Lupin is indicated for the long term maintenance treatment of bronchospasm and dyspnoea associated with chronic obstructive pulmonary disease (COPD). Tiotropium Lupin is indicated for the prevention of COPD exacerbations.
4.2 Dose and Method of Administration
The delivered dose (the dose that leaves the mouthpiece of the LupinHaler) is 10 micrograms of tiotropium per capsule.
The recommended dosage of Tiotropium Lupin is inhalation of the contents of one capsule, once daily with the LupinHaler device, at the same time each day (see LupinHaler instructions for use in the patient information leaflet provided in each pack of Tiotropium Lupin).
Tiotropium Lupin capsules must not be swallowed.
Method of administration.
To ensure proper administration of the medicinal product the patient should be trained on how to use the inhaler by a physician or other healthcare professional. See LupinHaler instructions for use.
LupinHaler instructions for use. The LupinHaler enables you to inhale the medicine contained in the capsule that your physician has prescribed for your breathing problems.
Remember to carefully follow your doctor's instructions for using Tiotropium Lupin 18 microgram, inhalation powder. The LupinHaler is especially designed for the Tiotropium Lupin, capsule which contains the inhalation powder. You must not use the LupinHaler to take any other medication. You can use your LupinHaler until you have finished the medicine contained in this box.
LupinHaler components.
The LupinHaler is made of the following components:
1. Dust cap (lid).
2. Mouthpiece.
3. Mouthpiece grip.
4. Base.
5. Green piercing button.
6. Centre chamber.
7. Air Intake vents.
How to use the LupinHaler.
1. After removing LupinHaler device from the pouch, open the dust cap (lid) by pressing the green piercing button.
2. Pull the dust cap (lid) upwards away from the base to expose the mouthpiece. Open the mouthpiece by pulling the mouthpiece ridge up and away from the base so the centre chamber is showing. Then open the mouthpiece by pulling it upwards.
3. Remove a Tiotropium Lupin 18 microgram, inhalation powder capsule from the blister (only immediately before use, see Blister handling).
Place the capsule in the centre chamber. It does not matter which way the capsule is placed in the chamber.
4. Close the mouthpiece firmly against the transparent base until you hear a click. Leave the dust cap (lid) open.
5. Hold the LupinHaler device with the mouthpiece upwards and press the piercing button completely in only once, and release.
This makes holes in the capsule and allows the medication to be released when you breathe in.
6. Breathe out completely.
Important.
Please avoid breathing into the mouthpiece at any time.
7. Raise the LupinHaler to your mouth and close your lips tightly around the mouthpiece. Keep your head in an upright position and breathe in slowly and deeply but at a rate sufficient to hear or feel the capsule vibrate.
Breathe in until your lungs are full; then hold your breath as long as comfortable and at the same time take the LupinHaler out of your mouth. Resume normal breathing.
Repeat steps 6 and 7 once, in order to empty the capsule completely.
8. Open the mouthpiece again. Tip out the used capsule and dispose of it appropriately. As the empty capsule may still contain some powder, it should be disposed of carefully. Close the mouthpiece and dust cap for storage of your LupinHaler device.
Cleaning your LupinHaler.
Clean the LupinHaler once a month.
Open the dust cap and mouthpiece, then open the base by lifting the piercing button. Rinse all components of the LupinHaler completely with warm water to remove any powder.
Dry the LupinHaler thoroughly by tipping any excess water out on a paper towel and allowing the LupinHaler to air dry afterwards, leaving the dust cap, mouthpiece and base open. It takes 24 hours to air dry, so clean it right after you have used it and it will be ready for your next dose. If needed, the outside of the mouthpiece may be cleaned with a moist but not wet tissue.
Blister handling.
Tiotropium Lupin inhalation power capsules contain only a small amount of powder so that the capsule is only partially filled.
Follow the instructions below on how to remove a capsule from the blister.
1. Separate the blister strips by tearing along the perforation.
2. Only immediately before using the capsule, peel back foil at the "peel back" area indicated on the blister until one capsule is fully visible.
Remove capsule from blister pocket.
If another capsule is exposed to air inadvertently during this process, that capsule has to be discarded.
Special populations.
Elderly patients can use Tiotropium Lupin at the recommended dose.
Renally impaired patients can use Tiotropium Lupin at the recommended dose. However, as with all predominantly renally excreted drugs, Tiotropium Lupin use should be monitored closely in patients with moderate to severe renal impairment.
Hepatically impaired patients can use Tiotropium Lupin at the recommended dose.
Paediatric population.
There is no experience with Tiotropium Lupin in infants and children and therefore should not be used in this age group.4.3 Contraindications
Tiotropium Lupin is contraindicated in patients with a history of hypersensitivity to tiotropium bromide, atropine or its derivatives, e.g. ipratropium or oxitropium or to any other component of this product (see Section 6.1 List of Excipients; Section 4.4 Special Warnings and Precautions for Use).
4.4 Special Warnings and Precautions for Use
Tiotropium, as a once daily maintenance bronchodilator, should not be used for the treatment of acute episodes of bronchospasm, i.e. rescue therapy.
Immediate hypersensitivity reactions may occur after administration of tiotropium.
As with other anticholinergic drugs, tiotropium should be used with caution in patients with narrow-angle glaucoma, prostatic hyperplasia or bladder-neck obstruction. In a meta-analysis of placebo-controlled trials, tiotropium was associated with a non-significant increase in the risk of urinary retention, and a significant increase in the risk of micturition difficulties.
Inhaled medicines may cause inhalation-induced bronchospasm.
Tiotropium should be used with caution in patients with recent myocardial infarction < 6 months; any unstable or life-threatening cardiac arrhythmia or cardiac arrhythmia requiring intervention or a change in drug therapy in the past year; hospitalisation of heart failure (NYHA Class III or IV) within the past year. These patients were excluded from the clinical trials and these conditions may be affected by the anticholinergic mechanism of action.
As with all predominantly renally excreted drugs, tiotropium use should be monitored closely in patients with moderate to severe renal impairment (creatinine clearance of ≤ 50 mL/min) (see Section 5.2 Pharmacokinetic Properties).
Patients must be instructed in the correct administration of Tiotropium Lupin. Care must be taken not to allow the powder or spray to enter into the eyes. Eye pain or discomfort, blurred vision, visual halos or coloured images in association with red eyes from conjunctival congestion and corneal oedema may be signs of acute narrow-angle glaucoma. Should any combination of these symptoms develop, specialist advice should be sought immediately. Miotic eye drops are not considered to be effective treatment.
Tiotropium Lupin should not be used more frequently than once daily (see Section 4.9 Overdose).
Tiotropium Lupin capsules are to be used only with the LupinHaler device (see Section 4.2 Dose and Method of Administration).
Lactose monohydrate.
This product contains 5.5 mg of lactose monohydrate per capsule. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Use in hepatic impairment.
There are no data on the use of tiotropium in patients with hepatic impairment. As tiotropium is primarily cleared by renal mechanisms, no dosage adjustment is recommended. However, patients should be monitored closely.
Use in renal impairment.
Renally-impaired patients can use Tiotropium Lupin at the recommended dose. However, as with all predominantly renally excreted drugs, tiotropium use should be monitored closely in patients with moderate to severe renal impairment.
Use in the elderly.
Elderly patients can use Tiotropium Lupin at the recommended dose. Renal clearance of tiotropium is likely to be slower in elderly patients (see Use in renal impairment).
Paediatric use.
The safety and effectiveness of tiotropium in paediatric patients has not been established. Therefore, Tiotropium Lupin should not be used in paediatric patients.
Effects on laboratory tests.
No data available.4.5 Interactions with Other Medicines and Other Forms of Interactions
Although no formal drug interaction studies have been performed, tiotropium has been used concomitantly with other drugs which are commonly used in the treatment of COPD, including sympathomimetic bronchodilators, methylxanthines, oral and inhaled steroids without clinical evidence of drug interactions.
Common concomitant medications (LABA, ICS and their combinations) used by patients with COPD were not found to alter the exposure to tiotropium.
Limited information about co-administration of other anticholinergic medicines with tiotropium is available from a clinical trial. The concomitant use of tiotropium with other anticholinergic agents (e.g. glycopyrronium, aclidinium, umeclidinium, ipratropium) is expected to have additive anticholinergic effects. Acute single dose administration of ipratropium bromide after 19 days of tiotropium treatment in healthy volunteers (n=35) was not associated with relevant changes in vital signs or electrocardiographic findings. Adverse events were reported by 3 subjects (9%) in the study during ipratropium treatment with tiotropium compared to 1 subject (3%) during placebo treatment with tiotropium. Ipratropium was associated with a 16% decrease in salivary secretions in healthy volunteers. Chronic co-administration of other anticholinergic medicines with tiotropium has not been studied and is therefore not recommended.
4.6 Fertility, Pregnancy and Lactation
Effects on fertility.
Clinical data on fertility are not available for tiotropium. Tiotropium (as bromide) did not affect the fertility of male or female rats when administered by inhalation at doses up to 2 mg/kg (750x the maximum recommended human daily dose of 22.5 micrograms, based on body surface area).
(Category B1)
There is a limited amount of data from the use of tiotropium in pregnant women. Reproductive toxicity studies with tiotropium bromide administered by inhalation to rats and rabbits at doses up to 2.0 and 0.5 mg/kg/day, respectively, produced no evidence of foetal malformations. These doses correspond to 750x and 400x the maximum recommended human daily dose of 22.5 micrograms based on body surface area. Animal studies do not suggest direct or indirect harmful effects with respect to reproductive toxicity at clinically relevant doses.
As a precautionary measure, it is preferable to avoid the use of tiotropium during pregnancy.
Clinical data from lactating women exposed to tiotropium are not available. Based on studies in lactating rats, a small amount of tiotropium is excreted in breast milk.
Therefore, tiotropium should not be used in lactating women unless the expected benefit outweighs any possible risk to the infant.4.7 Effects on Ability to Drive and Use Machines
No studies on the effects on the ability to drive and use machines have been performed. The occurrence of dizziness or blurred vision may influence the ability to drive and use machinery.
4.8 Adverse Effects (Undesirable Effects)
Summary of the safety profile.
Many of the listed undesirable effects can be assigned to the anticholinergic properties of tiotropium.
Adverse drug reactions were identified from data obtained in clinical trials and spontaneous reporting during post approval use of the drug. The clinical trial database includes 9,647 tiotropium patients from 28 placebo-controlled clinical trials with treatment periods ranging between four weeks and four years, contributing 12,469 person years of exposure to tiotropium.
Frequency is defined using the following convention: Very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000); very rare (< 1/10,000); and not known (cannot be estimated from the available data). See Table 1.

Reporting suspected adverse effects.
Reporting suspected adverse reactions after registration of the medicinal product is important. It allows continued monitoring of the benefit-risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.tga.gov.au/reporting-problems.4.9 Overdose
For information on the management of overdose, contact the Poison Information Centre on 13 11 26 (Australia).
High doses of tiotropium may lead to anticholinergic signs and symptoms.
However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 282 micrograms tiotropium in healthy volunteers. Additionally, no relevant adverse effects, beyond dry mouth, were observed following 7-day dosing of up to 141 micrograms tiotropium in healthy volunteers. In a multiple dose study in COPD patients, with a maximum daily dose of 36 micrograms tiotropium over four weeks, no significant undesirable effects were observed.
Acute intoxication by inadvertent oral ingestion of tiotropium powder is unlikely, due to low oral bioavailability.
5 Pharmacological Properties
5.1 Pharmacodynamic Properties
Pharmacotherapeutic group: Other drugs for obstructive airway diseases, inhalants, anticholinergics; ATC code: R03BB04.
Mechanism of action.
Tiotropium is a long-acting, specific antimuscarinic (anticholinergic) agent. It has similar affinity to the muscarinic receptor subtypes M1 to M5 (KD 5-41 pM). In the airways, inhibition by tiotropium of M3-receptors at the smooth muscle results in relaxation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors. In non-clinical in vitro as well as in vivo studies, bronchoprotective effects were dose-dependent. Bronchoprotective effects lasting at least 24 hours were observed in some of the in vivo studies. The long duration of effect of tiotropium is likely to be due to its slow dissociation from M3-receptors. Tiotropium exhibited a significantly longer dissociation half-life from M3-receptors than ipratropium.
Tiotropium, a N-quaternary anticholinergic agent, is topically (broncho-) selective when administered by inhalation. The high potency (IC50 approximately 0.4 nanoM for M3) and slow receptor dissociation is associated with a significant and long-acting bronchodilation in patients with COPD.
The bronchodilation following inhalation of tiotropium is primarily a local effect on the airways, not a systemic one.
Cardiac electrophysiology.
In a dedicated QT study involving 53 healthy volunteers, tiotropium 18 micrograms and 54 micrograms (i.e. three times the therapeutic dose) over 12 days did not significantly prolong QT intervals of the ECG.
Clinical trials.
Clinical efficacy. The pivotal clinical development program consisted of four one-year randomised, double-blind studies; two placebo-controlled and two with an active control (ipratropium) in 1,456 COPD patients, 906 of which received tiotropium. The studies assessed lung function in terms of forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and peak expiratory flow rate (PEFR). Health outcome measures including dyspnoea, exacerbations, hospitalisations and health-related quality of life (as measured by the St George's Respiratory Questionnaire, SGRQ) were also assessed. In addition, the development program included two large trials of six months duration which compared the bronchodilator efficacy and safety of tiotropium, with salmeterol inhalation aerosol and placebo in patients with COPD. These two studies randomised a total of 1,207 patients, with approximately one-third treated with tiotropium.
Lung function. Overall results from the four one-year studies demonstrated that tiotropium, administered once daily, provided significant improvement in lung function (FEV1 and FVC) within 30 minutes following the first dose and that this improvement was maintained for 24 hours. Pharmacodynamic steady state was reached within one week, with near maximal bronchodilation observed by the third day. Tiotropium significantly improved morning and evening PEFR as measured by the patient's daily recordings. The bronchodilator effects of tiotropium were maintained throughout the one-year period of administration with no evidence of tolerance.
In the two one-year, randomised, double blind, placebo-controlled studies, 550 patients received tiotropium once daily and 371 patients received placebo. Mean differences in FEV1 between tiotropium and placebo were highly statistically significant at all time points (p < 0.0001). The mean trough FEV1 at Day 92 (defined as the primary efficacy endpoint) was 0.14 L greater following tiotropium than placebo (p < 0.0001) and remained significantly different from placebo throughout the one year observation period (p < 0.0001). The FVC response generally paralleled that of FEV1.
In the two one-year, randomised, double blind, ipratropium-controlled studies, 356 patients received tiotropium once daily and 179 patients received ipratropium (2 puffs of 20 micrograms) four times a day. Mean differences in trough FEV1 between tiotropium and ipratropium were highly statistically significant at all time points (p < 0.0001). The mean trough FEV1 on Day 92 was 0.14 L greater following tiotropium than ipratropium (p < 0.0001). The FVC response generally paralleled that of FEV1.
Long-term clinical trials (6 months and 1 year). Dyspnoea, exercise tolerance.
In the one-year trials, tiotropium significantly improved dyspnoea in patients, as evaluated using the Mahler Transitional Dyspnoea Index (TDI) and patient daily reported symptoms. Following treatment with tiotropium, the dyspnoea score improved significantly when compared to placebo, with changes in each domain, as well as the focal score, being highly statistically significant over one year (p < 0.0002). The proportion of patients treated with tiotropium who achieved a TDI focal score change of at least 1 point over the one-year period, representing a clinically meaningful difference, was statistically greater than the proportion of patients treated with placebo (p < 0.0001).
When compared to ipratropium, patients treated with tiotropium exhibited significantly less dyspnoea at each time point, and the proportion of patients achieving a difference of 1 point in the TDI focal score was significantly greater in the tiotropium group.
The impact of improvement in dyspnoea on functional activities was investigated in two randomised, double-blind, placebo-controlled, parallel group studies in 433 COPD patients. The studies investigated whether six weeks treatment with tiotropium once-daily improves exercise tolerance in patients with COPD as measured by symptom-limited exercise endurance time (ET) during constant work rate cycle ergometry at 75% of maximal work capacity. Results demonstrated that tiotropium significantly improved ET by 20% to 28% compared with placebo. Increases in ET (seconds) are shown in Table 2.
Additionally in these trials, tiotropium demonstrated significant reductions in lung hyperinflation at rest and significant reductions in lung hyperinflation and dyspnoea during constant work rate cycle exercise.

Health related quality of life.
The SGRQ was the primary instrument used to evaluate disease-specific health related quality of life, with the impact domain stated as the primary endpoint. Tiotropium was significantly more effective than both placebo and ipratropium in improving health-related quality of life based on the SGRQ. The percentages of patients in the tiotropium groups who demonstrated a clinically meaningful improvement (pre-specified criteria of 4 units) over baseline were significantly greater than those in the placebo and ipratropium groups.
Tiotropium was more effective than placebo in each domain. Generally, the difference between the treatment groups increased between baseline and the last treatment visit. For the primary measure, Impacts score, the difference between the two treatment groups ranged from 1.8 to 4.0 and was statistically significant (p < 0.05) on all test days.
A significantly greater (p < 0.05) percentage of patients in the tiotropium group showed a clinically meaningful improvement (drop of 4 units) in the Impacts score from six months through to the end of the study and for Total score from three months through to the end of the study.
Tiotropium was also shown to be more effective than ipratropium in improving health-related quality of life using the SGRQ. For Impacts score, the difference between the two treatment groups in the mean score ranged from 0.6 at 8 days to 4.3 at 364 days and was statistically significant from three months through the end of the study. Statistically significant differences between tiotropium and ipratropium were also noted for the Total score on four of six test days.
A significantly greater (p < 0.05) percentage of patients in the tiotropium group showed clinically meaningful improvement (difference greater than 4 units) in both Impacts and Total scores over ipratropium after six months.
Two trials of six months duration compared the bronchodilator efficacy and safety of tiotropium once daily with salmeterol inhalation aerosol (50 micrograms twice daily) and placebo in patients with COPD. In one study, designed to evaluate the 12-hour duration of action, when the effects over time for tiotropium and salmeterol were compared, the mean trough FEV1 in the tiotropium group was significantly higher than that in the salmeterol group (p < 0.05), beginning on day 57. The difference between tiotropium and salmeterol for trough, average and peak FEV1 response was statistically significant (p < 0.05), except for trough response on day 15, and average and peak FEV1 response on day 1. At the end of the study, trough FVC had improved in the tiotropium group significantly above the placebo (p < 0.001) and the salmeterol (p < 0.01) groups. At the end of the combined six months trials, the improvement in TDI focal scores for tiotropium above placebo was 1.1 units (p < 0.001), which was both statistically and clinically significant, and for salmeterol above placebo was 0.7 units (p < 0.05), which was not clinically significant.
COPD exacerbations.
In the analysis of the pooled data from the four one-year studies, tiotropium significantly reduced both the number of COPD exacerbations and the number of hospitalisations associated with COPD exacerbations. In addition, time to first COPD exacerbation and to first hospitalisation associated with a COPD exacerbation was significantly prolonged.
In the placebo-controlled trials, the percentage of patients with at least one exacerbation during the treatment period was 36% in the tiotropium group and 42% in the placebo group (p=0.03); at least one hospitalisation for exacerbation occurred in 5.5% and 9.4% of patients respectively (p=0.019). The number of exacerbations and hospitalisations associated with exacerbations (expressed as events per 100 patient years) were significantly fewer for patients treated with tiotropium compared to placebo (p=0.045 and p=0.019 respectively). Patients on tiotropium also spent significantly fewer days in hospital for exacerbations compared to placebo (p=0.023). The time to first exacerbation was significantly delayed in the tiotropium group relative to placebo (p=0.011). Overall, these data indicate that therapy with tiotropium is associated with a delayed onset and a lower incidence of COPD exacerbations.
In the ipratropium-controlled trials, the percentage of patients with an exacerbation during the treatment period was 35% in the tiotropium group and 46% in the ipratropium group, a difference that was statistically significant. A similar trend was seen for hospitalisations for exacerbation (7.3% vs 11.7%; p=0.108). The number of exacerbations and exacerbation days impacted by these events was also less in the tiotropium group compared to ipratropium (p=0.006 and p=0.002, respectively). A similar trend was observed for hospitalisations (p=0.0803) and hospitalisation days for exacerbations (p=0.86). The time to first exacerbation, as well as for hospitalisation for exacerbation, was significantly delayed in the tiotropium group relative to the ipratropium group (p=0.008 and 0.048, respectively). Overall, these data indicate that tiotropium is associated with reduced exacerbations.
Tiotropium significantly reduced the percentage of patients experiencing one or more COPD exacerbations compared with placebo in a six-month randomised, double-blind, placebo-controlled trial of 1,829 patients with COPD (27.9% vs 32.3%, respectively, p=0.0368). The mean number of exacerbations per patient-year was significantly lower in the tiotropium group compared to placebo (0.85 vs 1.05, respectively, p=0.003), as was the number of exacerbation days (p < 0.0001). Time to first exacerbation was significantly increased in the tiotropium group compared to placebo (relative risk=0.834, p=0.034). Fewer tiotropium patients were hospitalised because of COPD exacerbation (7.0% vs 9.5%, respectively; p=0.056), although this difference was not statistically significant. The number of hospitalisations for exacerbations per patient-year was significantly lower in the tiotropium group, compared to placebo (p=0.013). Similarly, the mean number of hospitalisations days for exacerbations was lower in the tiotropium group compared to placebo (1.43 vs 1.70 days per patient-year, p=0.0013). The time to first hospitalisation for an exacerbation was significantly increased in the tiotropium group compared to placebo (relative risk=0.723, p ≤ 0.05) (see Figure 1).
 A one-year randomised, double-blind, double-dummy, parallel-group trial compared the effect of treatment with 18 micrograms of tiotropium once daily with that of 50 micrograms of salmeterol HFA pMDI twice daily with the primary endpoint time to first moderate or severe exacerbation in 7,376 patients with COPD and a history of exacerbations in the preceding year (74.6% of treated patients were men, 99.6% white, and 48.1% current smokers; the mean age was 62.9 years and the mean FEV1 was 49.3% predicted). The treatment groups were balanced with respect to demographics, COPD characteristics, pulmonary medication use at baseline, and concomitant diagnoses. Patients were allowed to continue their usual medications for COPD, except for anticholinergic drugs and long-acting β2-agonists, during the double-blind treatment phase. Short-acting β2-agonists were also permitted, as necessary, as rescue medications for acute relief of COPD symptoms. See Figures 2 and 3, Table 3.
A one-year randomised, double-blind, double-dummy, parallel-group trial compared the effect of treatment with 18 micrograms of tiotropium once daily with that of 50 micrograms of salmeterol HFA pMDI twice daily with the primary endpoint time to first moderate or severe exacerbation in 7,376 patients with COPD and a history of exacerbations in the preceding year (74.6% of treated patients were men, 99.6% white, and 48.1% current smokers; the mean age was 62.9 years and the mean FEV1 was 49.3% predicted). The treatment groups were balanced with respect to demographics, COPD characteristics, pulmonary medication use at baseline, and concomitant diagnoses. Patients were allowed to continue their usual medications for COPD, except for anticholinergic drugs and long-acting β2-agonists, during the double-blind treatment phase. Short-acting β2-agonists were also permitted, as necessary, as rescue medications for acute relief of COPD symptoms. See Figures 2 and 3, Table 3.


 Compared with salmeterol, tiotropium increased the time to the first exacerbation (187 days vs 145 days), with a 17% reduction in risk (hazard ratio, 0.83; 95% CI, 0.77-0.90; p < 0.001). Tiotropium also increased the time to the first severe (hospitalised) exacerbation (hazard ratio, 0.72; 95% CI, 0.61-0.85; p < 0.001), reduced the annual number of moderate or severe (hospitalised) exacerbations (0.64 vs 0.72; rate ratio, 0.89; 95% CI, 0.83-0.96; p=0.002), and reduced the annual number of severe (hospitalised) exacerbations (0.09 vs 0.13; rate ratio, 0.73; 95% CI, 0.66-0.82; p < 0.001).
Compared with salmeterol, tiotropium increased the time to the first exacerbation (187 days vs 145 days), with a 17% reduction in risk (hazard ratio, 0.83; 95% CI, 0.77-0.90; p < 0.001). Tiotropium also increased the time to the first severe (hospitalised) exacerbation (hazard ratio, 0.72; 95% CI, 0.61-0.85; p < 0.001), reduced the annual number of moderate or severe (hospitalised) exacerbations (0.64 vs 0.72; rate ratio, 0.89; 95% CI, 0.83-0.96; p=0.002), and reduced the annual number of severe (hospitalised) exacerbations (0.09 vs 0.13; rate ratio, 0.73; 95% CI, 0.66-0.82; p < 0.001).
Long-term clinical trials (< 1 up to 4 years). In a 4-year trial of 5,993 patients, tiotropium did not alter the annualised rate of decline of FEV1 (primary endpoint), but maintained improvements in the secondary endpoint of the difference in FEV1 at clinic visits throughout 4 years (Figure 4).
 A significantly higher proportion of patients in the tiotropium group than in the placebo group had an improvement of ≥ 4 units in the secondary endpoint of SGRQ total scores (i.e. exceeded the minimal clinically important difference) from baseline at 1 year (49% vs 41%), 2 years (48% vs 39%), 3 years (46% vs 37%), and 4 years (45% vs 36%) (p < 0.001 for all comparisons).
A significantly higher proportion of patients in the tiotropium group than in the placebo group had an improvement of ≥ 4 units in the secondary endpoint of SGRQ total scores (i.e. exceeded the minimal clinically important difference) from baseline at 1 year (49% vs 41%), 2 years (48% vs 39%), 3 years (46% vs 37%), and 4 years (45% vs 36%) (p < 0.001 for all comparisons).
In the following secondary endpoints, tiotropium significantly delayed the time to the first exacerbation and significantly delayed the time to the first hospitalisation for an exacerbation. The Hazard Ratios (95% confidence interval [CI]) for an exacerbation or exacerbation leading to hospitalisation were 0.86 (0.81, 0.91) and 0.86 (0.78, 0.95), respectively. Tiotropium was also associated with a reduction in the mean number of exacerbations of 14% (p < 0.001). The mean numbers of exacerbations leading to hospitalisations were infrequent and did not differ significantly between the tiotropium and placebo groups.
During treatment, there was a 16% reduction in the risk of death. The incidence rate of death was 4.79 per 100 patient years in the placebo group vs 4.10 per 100 patient years in the tiotropium group (hazard ratio (tiotropium/placebo) = 0.84, 95% CI = 0.73, 0.97).
For the 4-year, protocol-defined study period up to day 1,440, the effect of tiotropium extended to end of treatment period. Among patients for whom vital-status information was available (95% of patients), 921 patients died: 14.4% in the tiotropium group and 16.3% in the placebo group (hazard ratio, 0.87; 95% CI, 0.76-0.99). During a period of 4 years plus 30 days (1,470 days) included in the intention-to-treat analysis, 941 patients died: 14.9% in the tiotropium group and 16.5% in the placebo group (hazard ratio, 0.89; 95% CI, 0.79-1.02). Fewer vital status data were available for the day 1,470 analyses (75% of patients). The effect became non-significant within the 30-day follow-up period, when according to protocol, patients were discontinued from their study medication.
Long-term tiotropium active-controlled study.
A long term, large scale, randomised, double-blind, active-controlled study with an observation period up to 3 years has been performed to compare the efficacy and safety of tiotropium inhalation spray 2.5 micrograms and tiotropium powder for inhalation 18 micrograms (5,711 patients receiving tiotropium 2.5 microgram (2 puffs comprise one medicinal dose of 5 micrograms); 5,694 patients receiving tiotropium 18 micrograms). The primary endpoints were time to first COPD exacerbation, time to all-cause mortality and in a sub-study (906 patients) trough FEV1 (pre-dose).
The time to first COPD exacerbation was similar during the study with tiotropium inhalation spray 2.5 micrograms and tiotropium powder for inhalation 18 micrograms (hazard ratio (tiotropium 2.5 micrograms/tiotropium 18 micrograms) 0.98 with a 95% CI of 0.93-1.03). The median number of days to the first COPD exacerbation was 756 days for tiotropium 2.5 micrograms and 719 days for tiotropium 18 micrograms.
The bronchodilator effect of tiotropium 2.5 micrograms was sustained over 120 weeks, and was similar to tiotropium 18 micrograms. The mean difference in trough FEV1 for tiotropium 2.5 micrograms versus tiotropium 18 micrograms was -0.010 L (95% CI -0.038-0.018 L).
All-cause mortality was similar during the study with tiotropium 2.5 micrograms and tiotropium 18 micrograms (hazard ratio (tiotropium 2.5 micrograms/tiotropium 18 micrograms) 0.96 with a 95% CI of 0.84-1.09).
5.2 Pharmacokinetic Properties
Tiotropium is a non-chiral quaternary ammonium compound and is sparingly soluble in water. Tiotropium is administered by dry powder inhalation. Generally, with the inhaled route of administration, the majority of the delivered dose is swallowed and deposited in the gastrointestinal tract, and to a lesser extent is delivered to the lungs.
Absorption.
Following inhalation in young healthy volunteers, the absolute bioavailability of 19.5% suggests that the proportion reaching the lung is highly bioavailable. The bioavailability is the apparent bioavailability, which is dependent upon the amount of tiotropium that is effectively inhaled. It is expected from the chemical structure of the compound that tiotropium is poorly absorbed from the gastro-intestinal tract. This was confirmed in a study in young healthy volunteers, with a low bioavailability of 2-3% for oral solutions. Food is not expected to influence the absorption of tiotropium for the same reason. Maximum tiotropium plasma concentrations were observed 5-7 minutes after inhalation. At steady state, peak tiotropium plasma concentrations in patients with COPD were 12.9 picogram/mL and decreased rapidly in a multi-compartmental manner. Steady state trough plasma concentrations were 1.71 picogram/mL.
Distribution.
Studies in rats have shown that tiotropium does not penetrate the blood-brain barrier to any relevant extent. Tiotropium has a plasma protein binding of 72% and shows a volume of distribution of 32 L/kg.
Metabolism.
Metabolism does not occur to any great extent in young healthy volunteers, as indicated by 74% renal excretion of unchanged drug after an intravenous dose. The major metabolic pathway is non-enzymatic ester cleavage to the alcohol N-methylscopine and dithienylglycolic acid that are inactive on muscarinic receptors.
In vitro metabolism.
In studies in animals and in vitro experiments with human liver microsomes and hepatocytes, minor amounts of a variety of glutathione conjugates, after oxidation of the thiophene rings, were observed. In vitro studies in human liver microsomes revealed that the enzymatic pathway, relevant for only a small amount of tiotropium metabolism, can be inhibited by cytochrome P450 (CYP) 2D6 inhibitor quinidine and CYP 3A4 inhibitors ketoconazole and gestodene.
Tiotropium, even in supra-therapeutic concentrations, does not inhibit CYP 1A1, 1A2, 2B6, 2C9, 2C19, 2D6, 2E1 or 3A in human liver microsomes.
Excretion.
The effective half-life of tiotropium ranges between 27 to 45 h following inhalation by patients with COPD. Total clearance was 880 mL/min after an intravenous dose in young healthy volunteers. Urinary excretion of unchanged substance in young healthy volunteers is 74% of an intravenous dose. Following inhalation of tiotropium by patients with COPD to steady state, urinary excretion is 7% (1.3 micrograms) of the unchanged dose over 24 hours, the remainder being mainly non-absorbed drug in the gut that is eliminated via the faeces. The renal clearance of tiotropium exceeds the creatinine clearance, indicating secretion into the urine. After chronic, once daily inhalation by patients with COPD, pharmacokinetic steady state was reached by day 7, with no accumulation thereafter.
Tiotropium demonstrates linear pharmacokinetics in the therapeutic range independent of the formulation.
Special populations.
Elderly patients.
As expected for all predominantly renally excreted drugs, advancing age was associated with a decrease of tiotropium renal clearance (365 mL/min in patients with COPD < 65 years to 271 mL/min in patients with COPD > 65 years). This did not result in a corresponding increase in AUC0-6,ss or Cmax,ss values.
Renally impaired patients.
Following once daily inhaled administrations of tiotropium to steady-state in patients with COPD with mild renal impairment (CLCR 50-80 mL/min) resulted in slightly higher AUC0-6,ss (between 1.8-30% higher) and similar Cmax,ss values compared to patients with COPD with normal renal function (CLCR > 80 mL/min). In patients with COPD with moderate to severe renal impairment (CLCR < 50 mL/min), the intravenous administration of tiotropium resulted in a doubling of the plasma concentrations (82% increase in AUC0-4h) and 52% higher Cmax compared to patients with COPD with normal renal function, which was confirmed by plasma concentrations after dry powder inhalation.
Hepatically impaired patients.
There are no data on the pharmacokinetics of tiotropium in hepatic impairment. Liver insufficiency is not expected to have any relevant influence on tiotropium pharmacokinetics. Tiotropium is predominantly cleared by renal elimination (74% in young healthy volunteers) and by simple non-enzymatic ester cleavage to products that do not bind to muscarinic receptors.
5.3 Preclinical Safety Data
Genotoxicity.
Tiotropium (as bromide) did not exhibit any genotoxic effects in assays for gene mutation (bacteria and mammalian cells in vitro and in vivo mouse micronucleus test) or DNA damage (rat hepatocytes in vitro).
Carcinogenicity.
Long-term carcinogenicity studies in mice and rats, with tiotropium (as bromide) administered by inhalation, showed no evidence of neoplastic responses. The highest doses studied were approximately 0.8x (male mouse), 38x (female mouse) and 16x (rat) greater than the maximum recommended human daily dose of 22.5 micrograms, based on body surface area.6 Pharmaceutical Particulars
6.1 List of Excipients
Tiotropium Lupin includes the excipient lactose monohydrate.
6.2 Incompatibilities
Incompatibilities were either not assessed or not identified as part of the registration of this medicine.
6.3 Shelf Life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
After first use, you can use your LupinHaler for up to 30 days to take your medication.
6.4 Special Precautions for Storage
Store below 25°C. Do not freeze. Avoid storage in direct sunlight or heat.
6.5 Nature and Contents of Container
Tiotropium Lupin capsules are presented in aluminium/aluminium blister packs.
Tiotropium Lupin is available in a carton combination pack containing 30 capsules and the LupinHaler device.
6.6 Special Precautions for Disposal
In Australia, any unused medicine or waste material should be disposed of by taking to your local pharmacy.
6.7 Physicochemical Properties
Tiotropium bromide is a white to yellowish-white, odourless crystalline powder. It exists as a quaternary ammonium salt, and there are no ionisable functional groups on the molecule. The active substance is not optically active.
Tiotropium bromide is freely soluble in dimethyl sulfoxide, soluble in methanol, sparingly soluble in water and practically insoluble in methylene chloride. The solubility in aqueous solutions at room temperature is approximately 2.5%, independent of pH. At pH 7.4, the apparent partition coefficient (log Papp) is -2.25.
A monohydrate form of tiotropium bromide is produced by the synthetic process. The compound melts with decomposition between 225°C and 235°C, when determined by differential scanning calorimetry at a heating rate of 10 K per minute.
Chemical structure.
 Chemical name: 3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane,7-[(hydroxydi-2-thienylacetyl)oxy]- 9,9-dimethyl-, bromide, monohydrate, (1α, 2β, 4β, 5α, 7β)-.
Chemical name: 3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane,7-[(hydroxydi-2-thienylacetyl)oxy]- 9,9-dimethyl-, bromide, monohydrate, (1α, 2β, 4β, 5α, 7β)-.
Molecular Formula: C19H22NO4S2Br.H2O.
Molecular Weight: 490.4 (monohydrate).
CAS Number.
139404-48-1.7 Medicine Schedule (Poisons Standard)
S4 - Prescription Only Medicine.



 A one-year randomised, double-blind, double-dummy, parallel-group trial compared the effect of treatment with 18 micrograms of tiotropium once daily with that of 50 micrograms of salmeterol HFA pMDI twice daily with the primary endpoint time to first moderate or severe exacerbation in 7,376 patients with COPD and a history of exacerbations in the preceding year (74.6% of treated patients were men, 99.6% white, and 48.1% current smokers; the mean age was 62.9 years and the mean FEV1 was 49.3% predicted). The treatment groups were balanced with respect to demographics, COPD characteristics, pulmonary medication use at baseline, and concomitant diagnoses. Patients were allowed to continue their usual medications for COPD, except for anticholinergic drugs and long-acting β2-agonists, during the double-blind treatment phase. Short-acting β2-agonists were also permitted, as necessary, as rescue medications for acute relief of COPD symptoms. See Figures 2 and 3, Table 3.
A one-year randomised, double-blind, double-dummy, parallel-group trial compared the effect of treatment with 18 micrograms of tiotropium once daily with that of 50 micrograms of salmeterol HFA pMDI twice daily with the primary endpoint time to first moderate or severe exacerbation in 7,376 patients with COPD and a history of exacerbations in the preceding year (74.6% of treated patients were men, 99.6% white, and 48.1% current smokers; the mean age was 62.9 years and the mean FEV1 was 49.3% predicted). The treatment groups were balanced with respect to demographics, COPD characteristics, pulmonary medication use at baseline, and concomitant diagnoses. Patients were allowed to continue their usual medications for COPD, except for anticholinergic drugs and long-acting β2-agonists, during the double-blind treatment phase. Short-acting β2-agonists were also permitted, as necessary, as rescue medications for acute relief of COPD symptoms. See Figures 2 and 3, Table 3.

 Compared with salmeterol, tiotropium increased the time to the first exacerbation (187 days vs 145 days), with a 17% reduction in risk (hazard ratio, 0.83; 95% CI, 0.77-0.90; p < 0.001). Tiotropium also increased the time to the first severe (hospitalised) exacerbation (hazard ratio, 0.72; 95% CI, 0.61-0.85; p < 0.001), reduced the annual number of moderate or severe (hospitalised) exacerbations (0.64 vs 0.72; rate ratio, 0.89; 95% CI, 0.83-0.96; p=0.002), and reduced the annual number of severe (hospitalised) exacerbations (0.09 vs 0.13; rate ratio, 0.73; 95% CI, 0.66-0.82; p < 0.001).
Compared with salmeterol, tiotropium increased the time to the first exacerbation (187 days vs 145 days), with a 17% reduction in risk (hazard ratio, 0.83; 95% CI, 0.77-0.90; p < 0.001). Tiotropium also increased the time to the first severe (hospitalised) exacerbation (hazard ratio, 0.72; 95% CI, 0.61-0.85; p < 0.001), reduced the annual number of moderate or severe (hospitalised) exacerbations (0.64 vs 0.72; rate ratio, 0.89; 95% CI, 0.83-0.96; p=0.002), and reduced the annual number of severe (hospitalised) exacerbations (0.09 vs 0.13; rate ratio, 0.73; 95% CI, 0.66-0.82; p < 0.001). A significantly higher proportion of patients in the tiotropium group than in the placebo group had an improvement of ≥ 4 units in the secondary endpoint of SGRQ total scores (i.e. exceeded the minimal clinically important difference) from baseline at 1 year (49% vs 41%), 2 years (48% vs 39%), 3 years (46% vs 37%), and 4 years (45% vs 36%) (p < 0.001 for all comparisons).
A significantly higher proportion of patients in the tiotropium group than in the placebo group had an improvement of ≥ 4 units in the secondary endpoint of SGRQ total scores (i.e. exceeded the minimal clinically important difference) from baseline at 1 year (49% vs 41%), 2 years (48% vs 39%), 3 years (46% vs 37%), and 4 years (45% vs 36%) (p < 0.001 for all comparisons). Chemical name: 3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane,7-[(hydroxydi-2-thienylacetyl)oxy]- 9,9-dimethyl-, bromide, monohydrate, (1α, 2β, 4β, 5α, 7β)-.
Chemical name: 3-Oxa-9-azoniatricyclo[3.3.1.02,4]nonane,7-[(hydroxydi-2-thienylacetyl)oxy]- 9,9-dimethyl-, bromide, monohydrate, (1α, 2β, 4β, 5α, 7β)-.