What is in this leaflet
Please read this leaflet carefully before you start using TRELEGY ELLIPTA.
This leaflet answers some common questions about TRELEGY ELLIPTA.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking TRELEGY ELLIPTA against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What TRELEGY ELLIPTA is used for
TRELEGY ELLIPTA is a dry powder inhaler. When you breathe in using the inhaler, the medicine is deposited into the lungs. TRELEGY ELLIPTA is used to treat asthma or chronic obstructive pulmonary disease (COPD).
Asthma is when the muscles surrounding the smaller airways become tight (bronchoconstriction), swollen and irritated (inflammation). Symptoms come and go and include shortness of breath, wheezing, chest tightness and cough.
COPD is a long-term condition that slowly gets worse. Symptoms include shortness of breath, cough, chest discomfort and coughing up mucus.
TRELEGY ELLIPTA contains three active ingredients: fluticasone furoate, umeclidinium (as bromide) and vilanterol (as trifenatate).
Fluticasone furoate belongs to a group of medicines called corticosteroids, often simply called steroids. They are not 'anabolic steroids' which are the steroids sometimes misused by athletes.
Corticosteroids are used to reduce inflammation. They reduce the swelling and irritation in the small air passages in the lungs and so ease breathing problems.
Umeclidinium and vilanterol belong to a group of medicines called bronchodilators. They work together to help open the airways and make it easier for air to get in and out of the lungs.
When TRELEGY ELLIPTA is used regularly, it can help to control the breathing difficulties related to your disease and help with the effects of the disease on your everyday life.
TRELEGY ELLIPTA should not be used to relieve a sudden attack of breathlessness or wheezing. If you get this sort of attack you must use a quick-acting inhaler (such as VENTOLIN).
Your doctor may have prescribed TRELEGY ELLIPTA for another reason.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
TRELEGY ELLIPTA should not be used in children or adolescents under the age of 18 years.
Before you use TRELEGY ELLIPTA
When you must not use it
Don't use TRELEGY ELLIPTA:
- if you are allergic (hypersensitive) to lactose or milk protein
- if you are allergic (hypersensitive) to fluticasone furoate, umeclidinium, vilanterol or any other ingredients of TRELEGY ELLIPTA (listed at the end of this leaflet).
If you think either of these applies to you, don't use TRELEGY ELLIPTA until you have checked with your doctor.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing, coughing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching, redness or hives on the skin
- suddenly feeling weak or light headed (may lead to collapse or loss of consciousness).
TRELEGY ELLIPTA contains lactose monohydrate.
If you have been diagnosed with an intolerance to some sugars, or to milk protein, talk to your doctor before you use TRELEGY ELLIPTA.
TRELEGY ELLIPTA is not usually recommended for use during pregnancy.
If you are pregnant, if you think you may be pregnant or if you are planning to have a baby, don't use TRELEGY ELLIPTA without asking your doctor. Your doctor will consider the benefit to you and the risk to your baby of taking TRELEGY ELLIPTA while you are pregnant.
If you are breast-feeding, check with your doctor before you take TRELEGY ELLIPTA. It is not known whether the ingredients of TRELEGY ELLIPTA can pass into breast milk.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to use it
Tell your doctor if you have or have had any of the following medical conditions:
- heart problems or high blood pressure
- liver disease, as you may be more likely to have side effects. If you have moderate or severe liver disease, your doctor will limit your dose to TRELEGY ELLIPTA 100/62.5/25 micrograms once daily.
- being or have been treated for tuberculosis (TB) or pneumonia
- eye problems such as glaucoma or cataracts
- an enlarged prostate, difficulty passing urine or a blockage in your bladder
- weak bones (osteoporosis)
- any type of viral, bacterial or fungal infection
- any other medical conditions
- a problem with your immune system.
Check with your doctor before you use TRELEGY ELLIPTA if you think any of these apply to you. Your doctor can discuss with you the risks and benefits involved.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop. This should include all of the medicines that you are using for your asthma or COPD.
Some medicines and TRELEGY ELLIPTA may interfere with each other. These include:
- medicines called beta blockers, to treat high blood pressure or other heart problems
- ketoconazole, to treat fungal infections
- ritonavir, to treat HIV
- other long acting medicines similar to this medicine that are used to treat breathing problems (i.e. inhalers used as maintenance or preventer therapy)
- medicines to treat depression or mood/mental disorders (such as monoamine oxidase inhibitors or tricyclics antidepressants).
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to use TRELEGY ELLIPTA
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions in the user leaflet, ask your doctor or pharmacist for help.
How much to use
Always use TRELEGY ELLIPTA exactly as your doctor has told you. Check with your doctor, nurse or pharmacist if you're not sure.
Don't use more than your doctor tells you to use.
Asthma
The dose for asthma is one inhalation of TRELEGY ELLIPTA 100/62.5/25 micrograms once daily OR one inhalation of TRELEGY ELLIPTA 200/62.5/25 micrograms once daily, at the same time each day. Your doctor will decide which strength of TRELEGY ELLIPTA is required to treat your asthma.
COPD
The dose for COPD is one inhalation of TRELEGY ELLIPTA 100/62.5/25 micrograms once daily at the same time each day.
How to use the inhaler
The full instructions for using TRELEGY ELLIPTA are given on a leaflet inside the pack. A brief summary of the instructions is provided below.
TRELEGY ELLIPTA is ready to use straight away. No preparation or checks of the inhaler are required.
Step 1: Prepare a dose
- Wait to open the cover until you are ready to take your dose. Slide the cover fully down until you hear a "click".

Do not open TRELEGY ELLIPTA until you are ready to take a dose.
Step 2: Inhale your medication
- Whilst holding the inhaler away from your mouth, breathe out as far as is comfortable.
- Put the mouthpiece between your lips, and close your lips firmly around it.

- Take one long, steady, deep breath in. Hold this breath for about 3-4 seconds or as long as is comfortable.
- Remove the inhaler from your mouth.
- Breathe out slowly and gently away from the mouthpiece.
After using TRELEGY ELLIPTA, you may clean the mouthpiece, using a dry tissue, before you close the cover. Do not immerse TRELEGY ELLIPTA in water.
Step 3: Close the inhaler and rinse your mouth
- Slide the cover upwards as far as it will go, to cover the mouthpiece.

When to use it
Use TRELEGY ELLIPTA regularly. It is very important that you use TRELEGY ELLIPTA every day, as instructed by your doctor. This will help to keep you free of symptoms throughout the day and night.
If you feel you are getting breathless or wheezy more often than normal, or if you are using a quick-acting inhaler (such as VENTOLIN) more than usual, see your doctor.
How long to use it
Use TRELEGY ELLIPTA for as long as your doctor recommends. It will only be effective as long as you are using it. Don't stop unless your doctor advises you to, even if you feel better.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and use your next dose when you are meant to. Otherwise, use it as soon as you remember, then go back to using it as you would normally.
Don't take an extra dose to make up for a missed dose.
If you are not sure what to do, ask your doctor or pharmacist.
If you become wheezy or breathless, use your quick-acting inhaler (e.g. VENTOLIN), then seek medical advice.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
In Australia, immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, if you think that you or anyone else may have taken too much TRELEGY ELLIPTA. Do this even if there are no signs of discomfort or poisoning.
If you have used larger doses than instructed for a long period of time, it is particularly important that you ask your doctor or pharmacist for advice. This is because larger doses of TRELEGY ELLIPTA may reduce the amount of steroid hormones produced naturally by your body.
While you are using TRELEGY ELLIPTA
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking TRELEGY ELLIPTA.
Contact your doctor if you experience a change in your vision.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor's appointments so that your progress can be checked.
Things you must not do
Do not take TRELEGY ELLIPTA to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
Check with your doctor if you are unsure about which medicines for asthma or COPD you should continue taking whilst being treated with TRELEGY ELLIPTA. You should stop taking any long-acting inhalers which contain inhaled corticosteroids or bronchodilators. However, you can continue to use a quick-acting inhaler (such as VENTOLIN) during an acute episode.
Things to be careful of
Be careful driving or operating machinery until you know how TRELEGY ELLIPTA affects you.
Side effects
Like all medicines, TRELEGY ELLIPTA can cause side effects, although not everybody gets them.
Your doctor will consider the risk of side effects when deciding which strength of TRELEGY ELLIPTA you should use.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Very common side effects
These may affect more than 1 in 10 people:
- common cold
Common side effects
These may affect up to 1 in 10 people:
- sore, raised patches in the mouth or throat caused by a fungal infection (thrush). Rinsing your mouth out with water immediately after using TRELEGY ELLIPTA may help stop this side effect developing
- headache
- cough
- hoarseness
- painful and frequent urination (may be signs of a urinary tract infection)
- joint pain
- back pain
- constipation
- itchy, runny or blocked nose
- pain in the back of the mouth and throat
- inflammation of the sinuses
- inflammation of the lungs (bronchitis)
- flu (influenza)
- infection of the nose, sinuses or throat
- infection of the upper airways
- infection of the lungs (pneumonia)*
*affected approximately 1 in 50 people in COPD clinical trials. In general, risk factors for pneumonia in people with COPD receiving inhaled medicines containing a corticosteroid include smoking, prior pneumonia, low body weight and severe COPD.
Uncommon side effects
These may affect up to 1 in 100 people:
- irregular heart beat
- faster heart beat
- dry mouth
- weakening of the bones, leading to fractures
- taste disturbance
- blurred vision
- eye pain
- glaucoma.
Rare side effects
These may affect up to 1 in 1000 people:
- allergic reactions
- difficulties passing urine (urinary retention)
- pain or discomfort passing urine (dysuria)
- awareness of heart beat (palpitations)
- anxiety
- tremor
- muscle spasms
- increase in blood sugar (hyperglycaemia)
Allergic reactions
Allergic reactions to TRELEGY ELLIPTA are rare (they affect less than 1 person in 1000). If you think you are having an allergic reaction to TRELEGY ELLIPTA, stop using this medicine and tell your doctor immediately or go the accident and emergency department at your nearest hospital. Symptoms of an allergic reaction usually include some or all of the following:
- shortness of breath
- wheezing, coughing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body (angioedema)
- rash, itching, redness or hives on the skin (urticaria)
- suddenly feeling weak or light headed (may lead to collapse or loss of consciousness).
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
After using TRELEGY ELLIPTA
Storage
Do not use TRELEGY ELLIPTA after the expiry date shown on the pack.
Store in the original package container in order to protect from moisture and do not open the foil lid until ready to inhale for the first time.
Safely throw away TRELEGY ELLIPTA one month after you open the foil tray or when the counter reads "0", whichever comes first. Write the date the inhaler should be discarded on the label in the space provided. The date should be added as soon as the inhaler has been removed from the tray.
Keep your inhaler in a cool dry place where the temperature stays below 30°C.
If you store in a refrigerator allow the inhaler to return to room temperature for at least an hour before use.
Do not store TRELEGY ELLIPTA or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
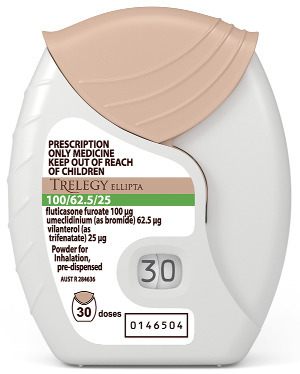
Product description
What it looks like
TRELEGY ELLIPTA is inhaled through the mouth using the ELLIPTA device. The active ingredients are in separate blisters in powder form inside the device. There are either 14 or 30 blisters on each strip, and so each device contains either 14 or 30 doses depending on which pack size or strength you have been given.
The ELLIPTA device itself is a plastic inhaler with a light grey body, a beige mouthpiece cover and a dose counter. It is packaged in a foil laminate tray with a peelable lid foil. The tray contains a desiccant sachet, to reduce moisture in the packaging. Once you have opened the lid of the tray, throw the desiccant away - do not open, eat or inhale it.
Ingredients
The active ingredients in TRELEGY ELLIPTA are fluticasone furoate, umeclidinium (as bromide) and vilanterol (as trifenatate).
Each dose contains either:
100 micrograms of fluticasone furoate, 62.5 micrograms of umeclidinium (as bromide) and 25 micrograms of vilanterol (as trifenatate)
or
200 micrograms of fluticasone furoate, 62.5 micrograms of umeclidinium (as bromide) and 25 micrograms of vilanterol (as trifenatate).
TRELEGY ELLIPTA also contains the inactive ingredients:
- lactose monohydrate
- magnesium stearate.
Supplier
TRELEGY ELLIPTA is supplied in Australia by:
GlaxoSmithKline Australia Pty Ltd
Level 4, 436 Johnston Street,
Abbotsford, Victoria 3067,
Australia.
Where to go for further information
Pharmaceutical companies are not in a position to give people an individual diagnosis or medical advice. Your doctor or pharmacist is the best person to give you advice on the treatment of your condition. You may also be able to find general information about your disease and its treatment from patient information groups and books, for example in public libraries.
Information on asthma is available from the website of the National Asthma Council Australia (www.nationalasthma.org.au).
Information on COPD is available from the website of the Lung Foundation Australia (https://lungfoundation.com.au/).
A copy of this CMI is available from the GSK Australia website (www.gsk.com.au/trelegy).
The information provided applies only to TRELEGY ELLIPTA.
TRELEGY ELLIPTA 100/62.5/25: AUST R 284636
TRELEGY ELLIPTA 200/62.5/25: AUST R 335858
Trade marks are owned by or licensed to the GSK group of companies.
© 2023 GSK group of companies or licensor.
This leaflet was prepared in June 2023.
Version 7.0
Published by MIMS July 2023


 Your medicine is now ready to be inhaled.
Your medicine is now ready to be inhaled. Take one long, steady, deep breath in. Hold this breath for about 3-4 seconds or for as long as is comfortable.
Take one long, steady, deep breath in. Hold this breath for about 3-4 seconds or for as long as is comfortable. Rinse your mouth with water without swallowing after you have used the inhaler.
Rinse your mouth with water without swallowing after you have used the inhaler.
 In a subset of subjects, in addition to adverse events reported in Table 2, adverse events occurring at a rate of greater than or equal to 1% in subjects receiving fluticasone furoate/umeclidinium/vilanterol for up to 52 weeks (n = 210) were viral respiratory tract infection, oropharyngeal pain and hypertension.
In a subset of subjects, in addition to adverse events reported in Table 2, adverse events occurring at a rate of greater than or equal to 1% in subjects receiving fluticasone furoate/umeclidinium/vilanterol for up to 52 weeks (n = 210) were viral respiratory tract infection, oropharyngeal pain and hypertension. Adverse reactions are listed by MedDRA system organ class and by frequency (see Table 4). Where adverse reaction frequencies differed between studies and populations, the higher frequency is reported. The following convention has been used for the classification of adverse reactions:
Adverse reactions are listed by MedDRA system organ class and by frequency (see Table 4). Where adverse reaction frequencies differed between studies and populations, the higher frequency is reported. The following convention has been used for the classification of adverse reactions:



 Moderate/severe asthma exacerbations were assessed over the 52-week treatment period (see Table 7). In the pooled analysis, the annualised rate of moderate/severe exacerbations was numerically lower with FF/UMEC/VI (100/62.5/25 and 200/62.5/25 microgram) compared with FF/VI (100/25 and 200/25 microgram) (13% reduction in rate; 95% CI: -5.2, 28.1). Descriptive analyses of unpooled treatment comparisons for the annualised rate of moderate/severe exacerbations are also provided.
Moderate/severe asthma exacerbations were assessed over the 52-week treatment period (see Table 7). In the pooled analysis, the annualised rate of moderate/severe exacerbations was numerically lower with FF/UMEC/VI (100/62.5/25 and 200/62.5/25 microgram) compared with FF/VI (100/25 and 200/25 microgram) (13% reduction in rate; 95% CI: -5.2, 28.1). Descriptive analyses of unpooled treatment comparisons for the annualised rate of moderate/severe exacerbations are also provided. In addition, severe asthma exacerbations were assessed. In a descriptive pooled analysis, a difference in the mean annualised rate of severe exacerbations was not observed for FF/UMEC/VI (100/62.5/25 and 200/62.5/25 microgram) compared with FF/VI (100/25 and 200/25 microgram) (2.6% reduction in rate; 95% CI: -26.2, 24.9).
In addition, severe asthma exacerbations were assessed. In a descriptive pooled analysis, a difference in the mean annualised rate of severe exacerbations was not observed for FF/UMEC/VI (100/62.5/25 and 200/62.5/25 microgram) compared with FF/VI (100/25 and 200/25 microgram) (2.6% reduction in rate; 95% CI: -26.2, 24.9). The ACQ-5 (comprising the 5 questions on symptoms from ACQ-7) results at Week 24 were similar to the ACQ-7 results. In a pooled descriptive analysis, the treatment difference for the ACQ-5 change from baseline for FF/UME/VI (100/62.5/25 and 200/62.5/25) compared with FF/VI (100/25 and 200/25) was -0.043 (-0.121, 0.035). The ACQ-5 responder rate was 64% for FF/UMEC/VI (100/62.5/25 and 200/62.5/25 microgram) compared with 60% for FF/VI (100/25 and 200/25 microgram) (OR: 1.23; 95% CI: 1.00, 1.52) at Week 24.
The ACQ-5 (comprising the 5 questions on symptoms from ACQ-7) results at Week 24 were similar to the ACQ-7 results. In a pooled descriptive analysis, the treatment difference for the ACQ-5 change from baseline for FF/UME/VI (100/62.5/25 and 200/62.5/25) compared with FF/VI (100/25 and 200/25) was -0.043 (-0.121, 0.035). The ACQ-5 responder rate was 64% for FF/UMEC/VI (100/62.5/25 and 200/62.5/25 microgram) compared with 60% for FF/VI (100/25 and 200/25 microgram) (OR: 1.23; 95% CI: 1.00, 1.52) at Week 24. The lung function, HRQoL, symptoms and exacerbations outcomes up to 52 weeks of treatment in a subset of patients (n = 430) were consistent with the results up to 24 weeks.
The lung function, HRQoL, symptoms and exacerbations outcomes up to 52 weeks of treatment in a subset of patients (n = 430) were consistent with the results up to 24 weeks. The effects on lung function (change from baseline trough FEV1) of FF/UMEC/VI compared with FF/VI and UMEC/VI for trough FEV1 were observed at all timepoints over the course of the 52-week study (see Figure 7).
The effects on lung function (change from baseline trough FEV1) of FF/UMEC/VI compared with FF/VI and UMEC/VI for trough FEV1 were observed at all timepoints over the course of the 52-week study (see Figure 7).



