What is in this leaflet
This leaflet answers some common questions about URSOFALK. It does not contain all of the available information. Reading this leaflet does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking URSOFALK against the benefits they expect it will have for you or your child.
If you have any concerns about taking URSOFALK, ask your doctor or pharmacist.
Keep this leaflet. You or your child may want to read it again.
What URSOFALK is used for
URSOFALK contains ursodeoxycholic acid. Ursodeoxycholic acid is a bile acid, which may have a protective effect on the liver by reducing the absorption of other potentially toxic bile salts.
URSOFALK may be used to treat liver diseases such as primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and cystic fibrosis (CF)-related cholestasis.
However, your doctor may prescribe this medicine for another use.
If you or your child wants more information, ask your doctor.
Ask your doctor if you or your child has any questions about why this medicine has been prescribed for you or your child.
URSOFALK is not addictive.
URSOFALK does not cause any negative effect on driving ability and operating machinery.
Before you or your child takes URSOFALK
URSOFALK is not suitable for everyone.
When you or your child must not take URSOFALK
Do not take URSOFALK if:
- you or your child is allergic to ursodeoxycholic acid or any other ingredients listed at the end of this leaflet
- you or your child has a bile duct or gall bladder that is swollen, painful or blocked
- the packaging is torn or shows signs of tampering
- the capsules, tablets or suspension, look to be deteriorating in any way
- the expiry date (EXP) printed on the pack has passed, as it may not work as well
- do not use URSOFALK suspension after 4 months of opening the bottle
If you are not sure whether you or your child should start taking this medicine, talk to your doctor.
Before you or your child starts to take it
Tell your doctor if you or your child:
- have allergies to any other medicines, foods, preservatives or dyes
- have kidneys that do not work properly
- have a gall bladder that cannot be seen on X-ray
- have calcified gallstones
- have a gall bladder which is not able to contract properly
- suffer from frequent cramp-like pains in the upper abdomen (biliary colic)
- is on a controlled sodium diet (for patients taking URSOFALK suspension)
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you or your child has not told your doctor any of the above, tell him/her before you or your child starts taking URSOFALK.
Taking other medicines
Tell your doctor or pharmacist if you or your child is taking any other medicines, including any that you or your child gets without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and URSOFALK may interfere with each other. These include:
- cholestyramine, or colestipol, medicines used to lower high levels of cholesterol in the blood
- an absorbent such as charcoal
- antacids or medicines used for indigestion that contains aluminium hydroxide and/or smectite (aluminium oxide)
- cyclosporine, medicine used to suppress the immune system
- ciprofloxacin and dapsone, an antibiotic used to prevent certain infections
- nitrendipine (used to treat high blood pressure) and other medicines which are metabolised in a similar way
- rosuvastatin
These medicines may be affected by URSOFALK or may affect how well URSOFALK works. You or your child may need different amounts of URSOFALK or at different times, or you or your child needs to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take URSOFALK
Take URSOFALK, or give URSOFALK to your child, as directed by your doctor or pharmacist. They may differ from the information contained in this leaflet.
If you or your child does not understand the instructions on the box/bottle, ask your doctor or pharmacist for help.
How much to take
The dose for URSOFALK is determined by your body weight. Your doctor should tell you how much URSOFALK you or your child should take.
Adults – the usual dose, depending on your weight, is as follows:
For PBC and chronic cholestatic liver diseases other than CF and PSC -
- Two to seven capsules or one to 31/2 tablets (or two to seven 5 mL spoonfuls of the suspension) per day.
For CF-related cholestasis -
- Three to nine capsules or 11/2 to 41/2 tablets (or three to nine 5 mL spoonfuls of the suspension) per day.
For PSC -
- one to nine capsules or 1/2 to 41/2 tablets (or one to nine 5 mL spoonfuls of the suspension) per day.
Children - the usual dose, depending on your child’s weight, is as follows:
- 1/4 to 11/2 spoonfuls (5 mL per spoonful) of the suspension per day.
Administration:
URSOFALK should be taken in divided doses, two to three times a day.
For PBC patients - during the first 3 months of treatment, you or your child should take URSOFALK capsules, tablets or suspension in two to three divided doses. With improvement of liver function tests, the daily dose may be taken in one single dose in the evening.
If you or your child is unsure of how much of the medicine you or your child should take, ask your doctor or pharmacist.
If you or your child has any questions about the prescribed dose, you or your child should ask your doctor or pharmacist.
How to take URSOFALK
URSOFALK capsules or tablets should be swallowed whole with a full glass of water because the content is bitter.
For URSOFALK suspension, shake the bottle well before use and accurately measure the dose with a medicine measure. Shaking the bottle and using a medicine measure will make sure that you or your child gets the correct dose. You can buy a medicine measure from your pharmacist.
Take URSOFALK capsules, tablets or suspension regularly.
When to take it
Take URSOFALK at about the same time each day. Taking it at the same time each day will have the best effect. It will also help you or your child to remember when to take it.
If you or your child needs to take a cholesterol lowering medicine or an antacid, take it at least 2 hours before or 2 hours after the dose of URSOFALK.
How long to take it
Continue taking the medicine for as long as your doctor tells you or your child to.
URSOFALK helps to control you or your child’s condition, but does not cure it. It is important to keep taking the medicine even if you or your child feels well.
If you are unsure whether you or your child should stop taking URSOFALK, talk to your doctor or pharmacist. You or your child may need to take URSOFALK for many months for it to work.
If you or your child forgets to take it
Take it as soon as you or your child remembers, and then go back to taking it as you or your child would normally.
If it is almost time for your next dose, skip the dose you or your child missed and take the next dose as you or your child would normally.
Do not take a double dose to make up for the dose that you or your child missed.
If you or your child is not sure what to do, ask your doctor or pharmacist.
If you or your child has trouble remembering to take, or give, the medicine, ask your pharmacist for some hints.
If you or your child takes too much
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have taken too much URSOFALK. Do this even if there are no signs of discomfort or poisoning. You or your child may need urgent medical attention.
Keep telephone numbers for these places handy.
Symptoms of an overdose may include diarrhoea. If you or your child suffers from diarrhoea, make sure you or your child drinks enough liquids to replace the fluid and electrolyte balance.
While you or your child is taking URSOFALK
Things you or your child must do
If you or your child is about to be started on any new medicine, remind your doctor and pharmacist that you or your child is taking URSOFALK.
Tell any other doctors, dentists, and pharmacists who treat you or your child that you or your child is taking this medicine.
If you or your child is going to have surgery, tell the surgeon or anaesthetist that you or your child is taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you or your child is about to have any blood tests, tell your doctor that you or your child is taking this medicine.
Keep all of your doctor’s appointments so that you or your child’s progress can be checked. Your doctor may do tests to assess you or your child’s liver function.
During the first three months of taking URSOFALK, your doctor should monitor you or your child’s liver function every 4 weeks. After the first three months of taking this medicine, your doctor should monitor you or your child’s liver function every 3 months.
See your doctor if you or your child feels that you or your child’s condition is not improving or is getting worse.
Things you or your child must not do
Do not take URSOFALK to treat any other complaints unless your doctor tells you or your child to.
Do not give your or your child’s medicine to anyone else, even if they have the same condition as you or your child.
Do not stop taking URSOFALK or change the dosage without checking with your doctor.
Side effects
Tell your doctor or pharmacist as soon as possible if you or your child does not feel well while taking URSOFALK.
Like all medicines, URSOFALK may occasionally cause side effects in some people. Sometimes they are serious, most of the time they are not. You or your child may need medical attention if you or your child gets some of the side effects.
Do not be alarmed by the following list of side effects. You or your child may not experience any of them.
Ask your doctor or pharmacist to answer any questions you or your child may have.
Side effects are normally mild, but if you or your child experiences the following effects, stop taking URSOFALK and tell your doctor immediately:
- Diarrhoea
- Itching/pruritus
- Urticaria (nettle rash)
- Allergic reactions
- Nausea/vomiting
- Sleep disturbance
- Pain in the stomach area or in the upper right part of the belly, under the ribs
During the treatment of primary biliary cholangitis, tell your doctor immediately if you or your child notices any of the following:
- Severe right-sided upper abdominal pain
During treatment of primary biliary cholangitis, stop taking URSOFALK if you have the following:
- Severe worsening (decompensation) of liver cirrhosis
Tell your doctor or pharmacist if you or your child experiences any other undesirable effects, particularly if they are severe or persistent.
After using URSOFALK
Storage
Keep the URSOFALK capsules, tablets or suspension in the blister pack/bottle until it is time to take them. If you or your child takes URSOFALK out of the pack/bottle they will not keep well.
Keep URSOFALK capsules, tablets or suspension in a cool, dark and dry place where the temperature stays below 25ºC.
Do not store URSOFALK or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep URSOFALK and all other medicines where children cannot reach them. A locked cupboard at least one and a half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you or your child to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
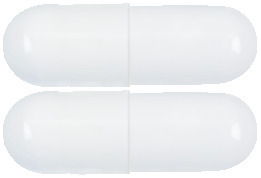
What URSOFALK looks like
URSOFALK Capsules are white, opaque, hard gelatin capsules. A box contains blister packs of 100 capsules.
URSOFALK Tablets are white, oblong tablets equipped with a breaking notch. A box contains blister of 50 or 100 tablets. Starter packs of 25 tablets are also available. Not all pack sizes are currently marketed in Australia.
URSOFALK Suspension is a white liquid containing small air bubbles, and has a lemon flavour (Giovaudan 87017). It comes in bottles of 250 mL.
Ingredients
The active ingredient in URSOFALK is ursodeoxycholic acid.
Each URSOFALK Capsule contains 250 mg of ursodeoxycholic acid. These capsules also contain maize starch, colloidal anhydrous silica, magnesium stearate, gelatin, titanium dioxide and sodium lauryl sulfate.
Each URSOFALK Tablet contains 500 mg ursodeoxycholic acid. These tablets also contain magnesium stearate, polysorbate 80, povidone, microcrystalline cellulose, colloidal anhydrous silica, crospovidone, purified talc, hypromellose and macrogol 6000.
Each 5 mL of the URSOFALK Suspension contains 250 mg of ursodeoxycholic acid. The suspension also contains benzoic acid, purified water, xylitol, glycerol, Avicel RC-591, propylene glycol, sodium citrate dihydrate, sodium cyclamate, citric acid, sodium chloride and 87017 lemon flavour.
All products are gluten free.
Australian Registration Number:
URSOFALK Capsules AUST R 66042
URSOFALK Suspension AUST R 75484
URSOFALK Tablets AUST R 262732
Sponsor
Dr Falk Pharma Australia Pty Ltd, 815 Pacific Highway, Chatswood, NSW 2067
URSOFALK® is a registered trademark of Dr Falk Pharma GmbH, Germany.
This leaflet was revised in May 2020
Published by MIMS December 2020