What is in this leaflet
This leaflet answers some common questions about valaciclovir. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking this medicine against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What this medicine is used for
Valaciclovir belong to a group of medicines called antivirals.
Valaciclovir is used for the following conditions in adults:
- Treatment of herpes zoster (shingles): It can reduce the duration of pain, length and severity of a shingles outbreak. Start the treatment within the first three days of the shingles attack.
- Treatment of ophthalmic zoster
(shingles affecting the eye region) - Treatment of genital herpes:
Valaciclovir can reduce the length and severity of a herpes outbreak, and reduce the duration of pain and time to healing of herpes blisters and crusts. It does not eliminate the herpes simplex virus (HSV) from the body. If you start taking it as soon as you feel an outbreak starting, you may prevent blisters from developing. - Prevention of genital herpes in immunocompromised patients with moderate to normal kidney function:
Valaciclovir can be taken by immunocompromised patients with adequate kidney function to help prevent the HSV infection coming back. - Reduction of transmission of genital herpes: Valaciclovir can reduce the risk of transmitting the HSV virus in patients who are taking it continuously. It does not cure genital herpes or eliminate the risk of transmission. Therefore, in addition to valaciclovir, it is recommended to avoid intercourse during a herpes outbreak and always use condoms to minimise the risk of spreading herpes to your partner.
- Prevention of cytomegalovirus infection (CMV) and disease:
Valaciclovir is used to prevent cytomegalovirus (CMV) infection and disease following solid organ transplantation. CMV is a type of herpes virus. It can cause symptoms similar to glandular fever (high temperature, sore throat and swollen glands).
How it works
Valaciclovir works by stopping the multiplication of the virus which causes shingles and herpes.
Ask your doctor if you have any questions about why valaciclovir has been prescribed for you. Your doctor may have prescribed it for another reason.
This medicine is not addictive.
This medicine is available only with a doctor's prescription.
There is not enough information to recommend the use of valaciclovir in children.
Before you take this medicine
When you must not take it
Do not take this medicine if you have an allergy to:
- valaciclovir
- acyclovir
- any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take Valaciclovir tablets if you become pregnant, trying to become pregnant or breastfeeding unless your doctor says you should. Your doctor will discuss the risks and benefits of using Valaciclovir when pregnant and during breastfeeding.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should be taking this medicine, talk to your doctor.
Before you start to take it
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- kidney or liver conditions
- are anaemic (reduced red blood cells or iron stores)
care should be taken to ensure adequate fluid intake in patients who are at risk of dehydration such as the elderly. - Previous skin reactions with valaciclovir which may include a rash, fever, facial swelling or blistering/peeling skin.
Tell your doctor if you are pregnant, intend to become pregnant or are breastfeeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell them before you start taking this medicine.
Taking other medicines
Tell your doctor and pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may interfere with valaciclovir. These include:
- mycophenolate
- ciclosporin
- tacrolimus
- aminoglycosides
- organoplatinum compounds
- iodinated contrast media
- methotrexate
- pentamidine
- foscarnet
These medicines may be affected by this medicine or may affect how well it works. You may need to use different amounts of your medicine or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking valaciclovir.
How to take this medicine
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
Your doctor or pharmacist will tell you:
- how many tablets to take at each dose
- how many doses to take each day
- when to take your doses each day
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
How much to take
Your doctor will decide what dose you should take and how often you should take valaciclovir. The dosage may vary depending on your medical history.
Prevention of cytomegalovirus infection (CMV) and disease: The usual dose for adults and children over 12 years of age is four 500mg tablets taken four times a day for 90 days.
If you have a kidney disease your doctor may reduce your dose.
For the treatment of shingles
500mg tablet: The normal dose to take is two tablets with water three times a day.
Treatment of herpes zoster: The usual dose is two tablets taken three times a day.
Treatment of genital herpes (first case): The usual dose is one tablet taken two times a day.
Treatment of genital herpes (recurrent cases): The usual dose is one tablet taken two times a day. You should start to take this medicine as early as possible if you think you are about to have an attack of genital herpes. Dosing should ideally start just before, or straight after the first signs of genital herpes infection appear.
Prevention of genital herpes in immunocompromised patients with moderate to normal kidney function: The usual dose is one tablet taken twice a day.
Reduction of transmission of genital herpes: In adults with normal immune function with less than 10 recurrences of genital herpes infection per year, the usual dose for the infected partner is one tablet taken once daily.
How to take it
Swallow the tablets whole with a full glass of water. You should drink plenty of fluids while taking valaciclovir, especially if elderly.
When to take it
Take your medicine at about the same time each day. Taking your tablets at the same time each day will have the best effect. It will also help you to remember when to take the tablets.
How long to take it
Treatment of herpes zoster: The usual course of treatment is 7 days.
Treatment of genital herpes infections: The usual course of treatment is 5 days. However, in some instances your doctor may want you to take your tablets for 10 days.
Prevention of genital herpes infections in immunocompromised patients with moderate to normal kidney function and reduction of transmission of the genital herpes infection: You should continue to take this medicine as long as prescribed by your doctor.
For the prevention of CMV infection and disease: The usual course of treatment is 90 days.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, then go back to taking it as you would normally.
Do not take a double dose to make up for the dose that you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) or go to Accident and Emergency at your nearest hospital if you think that you or anyone else has taken too much valaciclovir. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
While you are using this medicine
Things you must do
Drink plenty of fluids while you are taking valaciclovir.
If you are about to be started on any new medicine, tell your doctor and pharmacist that you are taking this medicine.
Tell any doctors, dentists or pharmacists that treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine. If may affect other medicines used during surgery.
If you become pregnant while taking valaciclovir, tell your doctor immediately.
Tell your doctor if for any reason you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
If you are about to have any blood test, tell your doctor that you are taking this medicine. It may interfere with the results of some tests.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
It is recommended that patients using Valaciclovir continuously, to prevent or reduce recurrent outbreaks or to reduce the risk of transmitting the virus that causes genital herpes also avoid contact when symptoms are present and always use condoms. Valaciclovir does not cure genital herpes or completely eliminate the risk of transmission. Because genital herpes is a sexually transmitted disease, you should minimise having intercourse when you have an outbreak of herpes or show any symptoms. This will avoid the risk of spreading herpes to your partner.
Things you must not do
Do not take valaciclovir to treat any other complaints unless your doctor says to.
Do not give this medicine to anyone else, even if they have the same condition as you.
Do not stop taking valaciclovir, or change the dose, without first checking with your doctor. If you stop too soon, the infection may start again.
Things to be careful of
Be careful driving or operating machinery until you know how valaciclovir affects you.
Side effects
Check with your doctor as soon as possible if you have any problems while taking this medicine, even if you do not think the problems relate to the medicine or are not listed in this leaflet.
Tell your doctor and pharmacist as soon as possible if you not feel well while you are taking valaciclovir.
This medicine helps most people, but it may cause unwanted some side effects in some people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by this list of possible side-effects. You may not experience any of them.
Ask your doctor or pharmacist any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- headache
- dry mouth
- fever, chills
- difficulty sleeping
- back pain
- nervousness
- skin rash which may be itchy
- stomach discomfort, such as vomiting, nausea, diarrhoea, constipation, pain, indigestion, flatulence
The above list includes the more common side effects of your medicine.
Tell your doctor as soon as possible if you notice any of the following. Some of these side effects are more common in patients with kidney disease or in those taking high doses of valaciclovir:
- sensitivity to UV light, such as developing a rash-like sunburn even after short exposure to UV light
- dizziness, confusion, imagining sights or sounds (hallucinations) difficulty in thinking
- drowsiness or decreased consciousness
- tiredness, dizziness or being short of breath when exercising (signs of anaemia)
If any of the following happen, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- wheezing, or difficulty in breathing; swelling of the lips/ mouth; hay fever; rash, hives, fainting (signs of an allergic reaction)
- hallucinations, confusion
- kidney damage
- unusual bruising or bleeding. Tell your doctor immediately if you notice any bruising or bleeding, as it may indicate that the number of platelets (a type of blood cell responsible for blood clotting) in your blood are reduced.
- Skin reactions which may include a rash, fever, facial swelling or blistering or peeling skin
- liver damage, which gets better when valaciclovir treatment is stopped.
- agitation or tremor
- Uncoordinated eye and muscle movements and speech or difficulty speaking
- Psychotic episodes
- Convulsions or seizures or coma
- Brain injury
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
Some of these side effects, for example, changes in kidney or liver function, can only be found when your doctor does test from time to time to check your progress.
After taking Valaciclovir APOTEX
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack, they may not keep well.
Keep your tablets in a cool, dry place where the temperature stays below 25°C.
Do not store this medicine or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where young children cannot reach it. A locked cupboard at least one-and- a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any tablets that are left over.
Product description
What it looks like
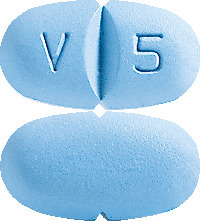
Blue coloured, capsule- shaped, biconvex, film-coated tablets, debossed with ‘V’ and ‘5’ on either side of the breakline on one side, notched on either side along with the breakline and plain on the other side. AUST R 177375.
Available in blister packs containing 10, 30, 42 or 100 tablets.
*Not all pack sizes may be available.
Ingredients
This medicine contains 500 mg of valaciclovir hydrochloride as the active ingredient.
It also contains the following:
- microcrystalline cellulose
- magnesium stearate
- crospovidone
- povidone (K 30)
- povidone (K 90D)
- indigo carmine aluminium lake
- Opadry 02C50740 Blue
Opadry 02C50740 Blue consists of:
- hypromellose
- titanium dioxide
- macrogol 400
- macrogol 6000
- polysorbate 80
- indigo carmine aluminium lake
This medicine does not contain sucrose, gluten, tartrazine or any other azo dyes.
Sponsor
Apotex Pty Ltd
16 Giffnock Avenue
Macquarie Park NSW 2113
This leaflet was prepared in February 2023
Published by MIMS March 2023

 For the prevention of herpes simplex in immunocompromised patients with creatinine clearance of > 15 mL/min, no dose modification is required.
For the prevention of herpes simplex in immunocompromised patients with creatinine clearance of > 15 mL/min, no dose modification is required. The creatinine clearance should be monitored frequently, especially during periods when renal function is changing rapidly e.g. immediately after transplantation or engraftment. The valaciclovir dosage should be adjusted accordingly.
The creatinine clearance should be monitored frequently, especially during periods when renal function is changing rapidly e.g. immediately after transplantation or engraftment. The valaciclovir dosage should be adjusted accordingly.




 There was no significant difference to the duration of zoster associated pain when treatment was started within 48 hours or 72 hours. Patients treated within 48 hours of rash onset were found to have faster healing rates as measured by the duration of new lesion formation and time to crusting or healing of 50% or more of lesions. Thus, greater benefit is gained if the drug is started within 48 hours. See Figure 1.
There was no significant difference to the duration of zoster associated pain when treatment was started within 48 hours or 72 hours. Patients treated within 48 hours of rash onset were found to have faster healing rates as measured by the duration of new lesion formation and time to crusting or healing of 50% or more of lesions. Thus, greater benefit is gained if the drug is started within 48 hours. See Figure 1. In a second, placebo controlled trial in patients under 50 years of age (n=399), demonstration of efficacy was restricted to a small decrease in mean time to cessation of new lesion formation. No significant effects were demonstrated for other outcomes of herpes zoster in this age group. Nevertheless, the occasional younger patients with severe herpes zoster may benefit from therapy with valaciclovir. Herpes zoster is usually a milder condition in younger patients.
In a second, placebo controlled trial in patients under 50 years of age (n=399), demonstration of efficacy was restricted to a small decrease in mean time to cessation of new lesion formation. No significant effects were demonstrated for other outcomes of herpes zoster in this age group. Nevertheless, the occasional younger patients with severe herpes zoster may benefit from therapy with valaciclovir. Herpes zoster is usually a milder condition in younger patients.
 Chemical formula: C13H20N6O4.HCl.
Chemical formula: C13H20N6O4.HCl.