What is in this leaflet
This leaflet answers some common questions about VALACICLOVIR RBX (valaciclovir).
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
This leaflet was last updated on the date at the end of this leaflet. More recent information may be available. The latest Consumer Medicine Information is available from https://www.ebs.tga.gov.au/ and may contain important information about the medicine and its use of which you should be aware.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking VALACICLOVIR RBX against the benefits it is expected to have for you.
If you have any concerns about using/ taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with this medicine. You may need to read it again.
What VALACICLOVIR RBX tablet is used for
VALACICLOVIR RBX tablets contain valaciclovir (as hydrochloride).
VALACICLOVIR RBX tablets belong to a group of medicines called anti-virals.
VALACICLOVIR RBX tablets are used for one or more of the following in adults:
- Treatment of Herpes zoster (shingles): Valaciclovir works by stopping the multiplication of the virus which causes shingles. It can reduce the length and severity of an outbreak of shingles and the duration of pain associated with shingles. It is important the treatment is started within the first three days of the shingles attack.
- Treatment of Ophthalmic zoster (shingles affecting the eye region)
- Treatment of genital herpes infection: Valaciclovir works by stopping the multiplication of the virus which causes herpes. It can reduce the length and severity of an outbreak of herpes, the duration of pain and shorten the time to healing of crusts associated with herpes. They do not eliminate the herpes virus from the body. The herpes virus is also known as the Herpes Simplex Virus, or HSV.
VALACICLOVIR RBX tablets help the blisters to heal more quickly. If you start taking them as soon as you feel an outbreak starting, you may actually prevent the blisters from developing. - Prevention of genital herpes in immunocompromised patients with moderate to normal kidney function: VALACICLOVIR RBX tablets can be taken by immunocompromised patients (with moderate to normal kidney function) to help prevent the HSV infection coming back. It will be determined by your doctor if you should be given VALACICLOVIR RBX tablets or not.
- Reduction of risk of transmission of genital herpes: VALACICLOVIR RBX can reduce the risk of transmitting the virus that causes genital herpes in patients who are taking it continuously. It does not cure genital herpes or completely eliminate the risk of transmission. Therefore, in addition to therapy with valaciclovir, it is recommended that patients avoid contact when symptoms are present and always use condoms.
Valaciclovir (or any other antiviral) is not a cure for genital herpes. Because genital herpes is a sexually transmitted disease, you should minimise having intercourse when you have an outbreak of herpes or show any symptoms. This will avoid the risk of spreading herpes to your partner. - Prevention of cytomegalovirus infection (CMV) and disease: VALACICLOVIR RBX tablets are used to prevent cytomegalovirus (CMV) infection and disease, following solid organ transplantation. CMV is a type of herpes virus. It can cause symptoms similar to glandular fever (high temperature, sore throat and swollen glands).
Ask your doctor if you have any questions about why VALACICLOVIR RBX tablets have been prescribed for you.
VALACICLOVIR RBX tablets are available only with a doctor's prescription.
Before you take VALACICLOVIR RBX tablets
When you must not take it
- Do not take VALACICLOVIR RBX tablets if you are allergic to valaciclovir, aciclovir or any of the inactive ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may be mild or severe. They usually include some or all of the following: wheezing, swelling of the lips/mouth, difficulty in breathing, hay fever, lumpy rash (hives) or fainting. - Do not take VALACICLOVIR RBX tablets after the expiry date (EXP) printed on the pack.
If you take it after the expiry date has passed, it may not work as well. - Do not take VALACICLOVIR RBX tablets if the packaging is torn or shows signs of tampering.
If you are not sure whether you should be taking VALACICLOVIR RBX tablets, talk to your doctor.
Before you start to take VALACICLOVIR RBX tablets
You must tell your doctor if:
- You are allergic to foods, dyes, preservatives or any other medicines.
- You have a kidney or liver problems.
- Do not take VALACICLOVIR RBX tablets if you are pregnant, intend to become pregnant or breast-feeding, unless your doctor says you should. Your doctor will discuss the risks and benefits of using VALACICLOVIR RBX tablets when pregnant and during breast-feeding.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines you buy without a prescription from a pharmacy, supermarket or health food shop.
Some medicines may interfere with the absorption or action of valaciclovir.
These include:
- Cimetidine (used for indigestion)
- Probenecid (used to treat joint disorders and used in combination with certain anti-infectives called antibiotics)
- Mycophenolate, Cyclosporin, Tacrolimus (used after organ transplantation)
These medicines may be affected by VALACICLOVIR RBX tablets, or may affect how well it works. You may need to use different amounts of your medicine or you may need to take different medicines. Your doctor or pharmacist will be able to tell you what to do when taking/being given VALACICLOVIR RBX tablets with other medicines.
Your doctor or pharmacist has more information on medicines to be careful with or avoid while taking VALACICLOVIR RBX tablets.
If you have not told your doctor about any of the above, please do so before you take VALACICLOVIR RBX tablets.
Use in children
There is not enough information to recommend the use of VALACICLOVIR RBX in children.
How to take VALACICLOVIR RBX tablets
How to take it
Take VALACICLOVIR RBX tablets exactly as your doctor has prescribed. Your doctor or pharmacist will tell you:
- how many tablets to take at each dose
- how many doses to take each day
- when to take your doses each day.
The label on the pack will give the same information. If there is something you do not understand, ask your doctor or pharmacist.
How much to take
Your doctor will decide what dose you should take and how often you should take VALACICLOVIR RBX tablets. The dosage may vary depending on your medical history.
Treatment of Herpes Zoster: the usual dose is 2 VALACICLOVIR RBX tablets (each containing 500 mg of valaciclovir) three times a day.
Treatment of genital herpes: If you are suffering from genital herpes infection for the first time, the dose usually given is one VALACICLOVIR RBX tablet (containing 500 mg of valaciclovir) given two times a day.
If you have had a herpes infection before, the dose usually given is one VALACICLOVIR RBX tablet (containing 500 mg of valaciclovir) given two times a day. You should start to take VALACICLOVIR RBX tablets as early as possible if you think you are about to have a recurrence (attack) of genital herpes. Dosing should ideally start just before, or straight after the first signs of genital herpes infection appear.
Prevention of genital herpes in immunocompromised patients with moderate to normal kidney function: To prevent the herpes infection appearing again, the usual dose to take is one VALACICLOVIR RBX tablet (containing 500 mg of valaciclovir) given two times a day.
Reduction of risk of transmission of genital herpes: In adults with normal immune function with less than 10 recurrences of genital herpes infection per year, the usual dose for the infected partner is one VALACICLOVIR RBX tablet (containing 500 mg of valaciclovir) taken once daily
Prevention of cytomegalovirus infection (CMV) and disease: The dosage of VALACICLOVIR RBX tablets in adults and adolescents (from 12 years of age) is 4 tablets (each containing 500 mg of valaciclovir) four times a day for 90 days.
If you are elderly: your dose may be adjusted by your doctor according to you kidney function.
If you have liver or kidney problems: Your dosage would be different if you have liver or kidney problems and your doctor will decide the dose and duration of treatment for you.
If you think you have been advised to take a different dose, talk to your doctor or pharmacist.
How to take it
VALACICLOVIR RBX tablets are to be taken orally. Swallow the tablet whole with a full glass of water.
You should drink plenty of fluids while on treatment with VALACICLOVIR RBX, especially if you are elderly.
When to take it
Take your VALACICLOVIR RBX tablets at about the same time each day. Taking your tablets at the same time each day will have the best effect. It will also help you to remember when to take the tablets.
How long to take it
For the treatment of shingles the usual course of treatment is 7 days.
For the treatment of genital herpes infections, the usual course of treatment is 5 days. However in some instances your doctor may want you to take your tablets for 10 days.
For prevention of genital herpes infections in immunocompromised patients with moderate to normal kidney function and reduction of risk of transmission of the genital herpes infection, you should continue to take this medicine as long as prescribed by your doctor.
For the prevention of CMV infection and disease, the usual course of treatment is 90 days.
Continue taking the medicine as long as the Doctor has told you to. Do not stop taking the VALACICLOVIR RBX tablets before the course of treatment is finished just because you feel better. If you stop too soon, the infection may start again.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to. Otherwise, take it as soon as you remember, then go back to taking it as you would normally.
Do not take a double dose to make up for the dose that you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (Overdose)
Immediately telephone your doctor or the Poisons Information Center (13 11 26), or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else has taken too much of VALACICLOVIR RBX tablets. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep this telephone number handy.
If you are not sure what to do, contact your doctor or pharmacist.
While you are taking VALACICLOVIR RBX tablets
Things you must do
- Tell your doctor or pharmacist that you are taking VALACICLOVIR RBX tablets if you are about to be started on any new medicines.
- Tell your doctor if you become pregnant or are trying to become pregnant or intend to breast-feed while you are taking VALACICLOVIR RBX tablets.
- Drink plenty of fluids while you are taking VALACICLOVIR RBX tablets.
Tell your doctor if, for any reason, you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Things you must not do
- Do not stop taking VALACICLOVIR RBX tablets, or alter the dose, without first checking with your doctor.
- Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
- Do not use VALACICLOVIR RBX tablets to treat any other complaints unless your doctor says to.
Things to be careful of
Be careful driving or operating machinery until you know how VALACICLOVIR RBX affects you.
Side effects
Check with your doctor as soon as possible if you have any health problems while taking VALACICLOVIR RBX tablets, even if you do not th the problems are connected with the medicine or are not listed in this leaflet.
Like all medicines, VALACICLOVIR RBX tablets can cause some side effects. If they occur, they are most likely to be minor and temporary. However, some may be serious and need medical attention.
Some people are allergic to medicines. Symptoms of an allergic reaction may be mild or severe. Allergic reactions to valaciclovir are rare. However, if you think you are having an allergic reaction, tell your doctor immediately or go to the casualty department at your nearest hospital. Symptoms usually include some or all of the following:
- Wheezing
- Swelling of the lips/mouth
- Difficulty in breathing
- Hay fever
- Lumpy rash (hives)
- Fainting
The most commonly reported side effects are:
- Headache
- Gastrointestinal discomfort (vomiting, nausea, diarrhoea, constipation, abdominal pain, indigestion, flatulence)
These should be reported to the doctor or pharmacist if they are severe or become troublesome.
Ask your doctor or pharmacist any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- Dry mouth
- Fever
- Difficulty sleeping
- Chills
- Back pain
- Nervousness
- Skin rash which may be itchy
- Weakness
You should contact your doctor if you experience any of the following side effects which are more common in patients with kidney disease or in those taking high doses of valaciclovir. Usually these side effects get better when valaciclovir treatment is stopped:
- Hallucinations
- Confusion
- Dizziness
- Drowsiness
Some rare side effects of valaciclovir include:
- Sensitivity to UV light, such as development of a rash like sunburn even after short exposure to UV light
- Damage to the kidney, which gets better when valaciclovir treatment is stopped.
- Unusual bruising or bleeding. Tell your doctor immediately if you notice any bruising or bleeding, as it may indicate that the number of platelets (a type of blood cell responsible for blood clotting) in your blood are reduced.
- Damage to the liver, which gets better when valaciclovir treatment is stopped.
This is not a complete list of all possible side effects. Others may occur in some people and there may be some side effects not yet known.
Ask your doctor or pharmacist any questions you may have.
Tell your doctor if you notice anything else that is making you feel unwell, even if it is not on this list.
Ask your doctor or pharmacist if you don't understand anything in this list.
Do not be alarmed by this list of possible side-effects. You may not experience any of them.
After using VALACICLOVIR RBX tablets
Storage
Keep your tablets in the blister pack until it is time to take them.
If you take the tablets out of the box or the blister pack they may not keep well.
Keep your VALACICLOVIR RBX tablets in a cool, dry place where it stays below 25°C.
Do not store it, or any other medicine, in the bathroom or near a sink.
Do not leave it in the car on hot days.
Heat and dampness can destroy some medicines.
Keep this medicine where young children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking VALACICLOVIR RBX tablets or you find that they have passed their expiry date, ask your pharmacist what to do with any tablets that are left over.
Product description
What it looks like
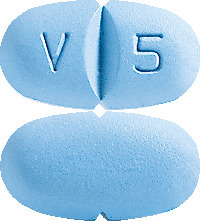
VALACICLOVIR RBX tablets are available as blue coloured, capsule-shaped, biconvex, film-coated tablets, debossed with ‘V’ and ‘5’ on either side of the breakline on one side, notched on either side along with the breakline and plain on the other side containing valaciclovir (as hydrochloride) 500 mg.
VALACICLOVIR RBX tablets are available in blister packs containing 30, 42 or 100 tablets.
Ingredients
Active ingredient:
Valaciclovir (as valaciclovir hydrochloride)
Inactive ingredients:
- microcrystalline cellulose
- magnesium stearate
- crospovidone
- povidone (K 30)
- povidone (K 90D)
- indigo carmine aluminium lake
- Opadry 02C50740 Blue
Opadry 02C50740 Blue consists of hypromellose, titanium dioxide, macrogol 400, macrogol 6000, polysorbate 80 and indigo carmine aluminium lake.
Sponsor
VALACICLOVIR RBX is supplied in Australia by:
Sun Pharma ANZ Pty Ltd.
Macquarie Park
NSW 2113
Australia
Australian Registration No.
AUST R 142803
This leaflet was prepared in June 2019.
Published by MIMS September 2019

 For the prevention of herpes simplex in immunocompromised patients with creatinine clearance of > 15 mL/min, no dose modification is required.
For the prevention of herpes simplex in immunocompromised patients with creatinine clearance of > 15 mL/min, no dose modification is required. The creatinine clearance should be monitored frequently, especially during periods when renal function is changing rapidly e.g. immediately after transplantation or engraftment. The valaciclovir dosage should be adjusted accordingly.
The creatinine clearance should be monitored frequently, especially during periods when renal function is changing rapidly e.g. immediately after transplantation or engraftment. The valaciclovir dosage should be adjusted accordingly.




 There was no significant difference to the duration of zoster associated pain when treatment was started within 48 hours or 72 hours. Patients treated within 48 hours of rash onset were found to have faster healing rates as measured by the duration of new lesion formation and time to crusting or healing of 50% or more of lesions. Thus, greater benefit is gained if the drug is started within 48 hours. See Figure 1.
There was no significant difference to the duration of zoster associated pain when treatment was started within 48 hours or 72 hours. Patients treated within 48 hours of rash onset were found to have faster healing rates as measured by the duration of new lesion formation and time to crusting or healing of 50% or more of lesions. Thus, greater benefit is gained if the drug is started within 48 hours. See Figure 1. In a second, placebo controlled trial in patients under 50 years of age (n = 399), demonstration of efficacy was restricted to a small decrease in mean time to cessation of new lesion formation. No significant effects were demonstrated for other outcomes of herpes zoster in this age group. Nevertheless, the occasional younger patients with severe herpes zoster may benefit from therapy with valaciclovir. Herpes zoster is usually a milder condition in younger patients.
In a second, placebo controlled trial in patients under 50 years of age (n = 399), demonstration of efficacy was restricted to a small decrease in mean time to cessation of new lesion formation. No significant effects were demonstrated for other outcomes of herpes zoster in this age group. Nevertheless, the occasional younger patients with severe herpes zoster may benefit from therapy with valaciclovir. Herpes zoster is usually a milder condition in younger patients. Valaciclovir was also significantly better than placebo in preventing or delaying the development of viraemia, viruria and clinical HSV disease during the study period. No valaciclovir recipient developed VZV disease, whereas 2% and 4% of placebo patients did, R+ and D+R- strata respectively. Additionally in D+R- patients, valaciclovir was shown to significantly reduce acute graft rejections (biopsy proven and clinical acute rejection by 57% and 45% respectively) and opportunistic infections (48% primarily bacterial and fungal infections). There were no significant differences in rates of chronic graft rejection. Allograft function and survival, including the proportion of patients with a functional graft at their last assessment were similar between treatment groups. Administration of valaciclovir was associated with significantly fewer hospital admissions and reduced use of ganciclovir and aciclovir for the treatment of CMV disease or other herpes virus infections, respectively.
Valaciclovir was also significantly better than placebo in preventing or delaying the development of viraemia, viruria and clinical HSV disease during the study period. No valaciclovir recipient developed VZV disease, whereas 2% and 4% of placebo patients did, R+ and D+R- strata respectively. Additionally in D+R- patients, valaciclovir was shown to significantly reduce acute graft rejections (biopsy proven and clinical acute rejection by 57% and 45% respectively) and opportunistic infections (48% primarily bacterial and fungal infections). There were no significant differences in rates of chronic graft rejection. Allograft function and survival, including the proportion of patients with a functional graft at their last assessment were similar between treatment groups. Administration of valaciclovir was associated with significantly fewer hospital admissions and reduced use of ganciclovir and aciclovir for the treatment of CMV disease or other herpes virus infections, respectively.
 Molecular formula: C13H20N6O4,HCl.
Molecular formula: C13H20N6O4,HCl.