What is in this leaflet
This leaflet answers some common questions about VALGANCICLOVIR VIATRIS tablets.
It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking VALGANCICLOVIR VIATRIS against the benefits expected for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What VALGANCICLOVIR VIATRIS is used for
VALGANCICLOVIR VIATRIS contains the active ingredient valganciclovir. In the body valganciclovir rapidly changes to ganciclovir.
VALGANCICLOVIR VIATRIS belongs to a class of medicines used to prevent the growth of viruses.
VALGANCICLOVIR VIATRIS acts against a virus called cytomegalovirus or CMV (a type of herpes virus). It prevents this virus from growing and multiplying in the body. CMV causes infections, mainly in people with poor immunity. Poor immunity can be caused by HIV/AIDS or by medications taken after an organ transplant.
VALGANCICLOVIR VIATRIS is used to treat CMV eye infections (known as CMV retinitis) in AIDS patients, which, if left untreated can cause blindness. It is not a cure for CMV eye infections.
VALGANCICLOVIR VIATRIS is not effective against any underlying HIV infection.
VALGANCICLOVIR VIATRIS is also used to prevent CMV infection in patients following organ transplantation.
Your doctor, however, may have prescribed VALGANCICLOVIR VIATRIS for another purpose.
Ask your doctor if you have any questions about why VALGANCICLOVIR VIATRIS has been prescribed for you.
VALGANCICLOVIR VIATRIS is not addictive.
This medicine is available only with a doctor's prescription.
Before you take VALGANCICLOVIR VIATRIS
Animal and other laboratory studies have shown VALGANCICLOVIR VIATRIS causes infertility, birth defects and cancer. It is possible that these effects may also occur in humans.
When you must not take it
Do not take VALGANCICLOVIR VIATRIS if you have had an allergy to VALGANCICLOVIR VIATRIS, ganciclovir or any ingredients at the end of this leaflet:
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not take VALGANCICLOVIR VIATRIS if you have very low blood counts for platelets (which help clotting), neutrophils (a type of white blood cell which defends against infection) or low haemoglobin (oxygen carrying substance in the blood).
Do not take VALGANCICLOVIR VIATRIS after the expiry date (EXP) printed on the pack has passed or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking VALGANCICLOVIR VIATRIS, talk to your doctor.
Use in children
There is limited information on the safety and effectiveness of the use of VALGANCICLOVIR VIATRIS in children. Your doctor will advise you whether VALGANCICLOVIR VIATRIS is suitable for your child.
Before you start to take it
Tell your doctor if:
- you are allergic to medicines such as aciclovir, valaciclovir, famciclovir, penciclovir. These medicines are from the same class as VALGANCICLOVIR VIATRIS and allergic reactions may occur.
- you are allergic to any other medicines, foods, dyes or preservatives, especially any medicine which you have taken previously to treat your current condition.
You have, any other health problems, especially the following:
- you have a history of low blood counts for platelets (thrombocytopenia), neutrophils (neutropenia) or anaemia
- you have, or previously have had, poor kidney function.
you are pregnant or intend to become pregnant.
- VALGANCICLOVIR VIATRIS is not recommended for use during pregnancy. VALGANCICLOVIR VIATRIS may affect your developing baby if you take it during pregnancy. Your doctor will discuss the risks and benefits of using VALGANCICLOVIR VIATRIS if you are pregnant.
you are breast-feeding or intend to breast-feed.
- It is not known whether VALGANCICLOVIR VIATRIS passes into breast milk. Breast-feeding is not recommended during therapy with VALGANCICLOVIR VIATRIS.
you are a woman who could become pregnant and you are not using contraception
- you must use a reliable form of contraception during VALGANCICLOVIR VIATRIS therapy, and for at least 30 days after stopping VALGANCICLOVIR VIATRIS, unless you are not sexually active.
you are a sexually active man
- you should use condoms during and for at least 90 days following treatment with VALGANCICLOVIR VIATRIS unless it is certain that your female partner is not at risk of pregnancy.
If you have not told your doctor about any of the above, tell him/her before you start taking VALGANCICLOVIR VIATRIS.
Taking other medicines
Tell your doctor or pharmacist if you are taking any other medicines, including any that you get without a prescription from a pharmacy, supermarket or health food shop.
Some medicines and VALGANCICLOVIR VIATRIS may interfere with each other. These medicines include:
- imipenem/cilastatin (Primaxin®), a combination of medicines used to treat some infections
- probenecid (e.g. Benemid®), a medicine used to treat gout
- zidovudine (AZT, Retrovir®, Combivir), didanosine (ddl or Videx®), stavudine, also known as D4T, other medicines used to treat HIV infection
- medicines for the treatment of cancer such as vincristine, adriamycin, hydroxyurea
- mycophenolate mofetil (CellCept®), and other medicines used to prevent organ rejection after a transplant e.g. ciclosporin, tacrolimus
- anti-infective agents such as trimethoprim / sulphonamides dapsone, pentamidine, flucytosine, pegylated interferons plus ribavirin and amphotericin B
These medicines may be affected by VALGANCICLOVIR VIATRIS, or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines.
Your doctor or pharmacist have more information on medicines to be careful with or avoid while taking VALGANCICLOVIR VIATRIS.
Ask your doctor or pharmacist if you are not sure about this list of medicines.
How to take VALGANCICLOVIR VIATRIS
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the bottle, ask your doctor or pharmacist for help.
How much to take
Take VALGANCICLOVIR VIATRIS exactly as your doctor has prescribed. Your doctor will tell you how many VALGANCICLOVIR VIATRIS tablets to take each day.
Treatment of CMV Retinitis in AIDS
- Induction Treatment for adults (for active CMV retinitis)
The usual dose is 900 mg (two 450 mg tablets) twice daily with food for 21 days. - Maintenance Treatment for adults (after induction treatment or for inactive CMV retinitis)
The usual dose is 900 mg (two 450 mg tablets) once daily with food.
Prevention of CMV Disease in Transplantation for adults
The usual dose is 900 mg (two 450 mg tablets) once daily with food, starting within 10 days post transplantation until 100 days post transplantation.
If you have received a kidney transplant, the same daily dose is required until 200 days post transplantation.
Your dose may have to be reduced or stopped if you have or develop low blood counts, have kidney disease, or if you are older than 65 years.
Prevention of CMV Disease in Transplantation for children
Your doctor will let you know how many VALGANCICLOVIR VIATRIS tablets you should give your child each day.
How to take it
Swallow tablets whole with a glass of water. VALGANCICLOVIR VIATRIS must be taken with food.
When to take it
Take VALGANCICLOVIR VIATRIS during or immediately after a meal. If you take VALGANCICLOVIR VIATRIS on an empty stomach, it may not work as well.
Take VALGANCICLOVIR VIATRIS at about the same time each day. Taking your medicine at the same time each day will have the best effect. It will also help you remember when to take VALGANCICLOVIR VIATRIS.
How long to take it for
Continue taking VALGANCICLOVIR VIATRIS until your doctor tells you to stop.
If you forget to take it
If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember and then go back to taking it as you would normally.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering your dose, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much VALGANCICLOVIR VIATRIS. Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
Keep telephone numbers for these places handy.
If you are not sure what to do, contact your doctor or pharmacist.
While you are taking VALGANCICLOVIR VIATRIS
Things you must do
Tell all doctors, dentists and pharmacists who treat you that you are taking VALGANCICLOVIR VIATRIS.
See your doctor regularly so that your CMV disease, blood cell counts and any other potential side effects may be monitored carefully. If blood cell counts are low then this may reduce your ability to fight infection, or for your blood to clot efficiently. If left undetected these effects on blood cells may contribute to death or serious illness.
If you have a CMV eye infection, you must also see your doctor regularly to monitor the condition of your retina (part of the eye).
If you become pregnant while taking VALGANCICLOVIR VIATRIS, tell your doctor immediately.
Condoms should be used by sexually active men while taking VALGANCICLOVIR VIATRIS and for 90 days after stopping treatment.
Women who are of childbearing potential should use contraception during and for at least 30 days after stopping VALGANCICLOVIR VIATRIS.
Tell your doctor if for any reason you have not taken your medicine exactly as prescribed. Otherwise, your doctor may think that it was not effective and change your treatment unnecessarily.
Keep all of your doctor's appointments so that your progress can be checked.
Things you must not do
Do not take VALGANCICLOVIR VIATRIS to treat any other complaints unless your doctor tells you to.
Do not give VALGANCICLOVIR VIATRIS to anyone else even if they have the same condition as you.
Do not stop taking VALGANCICLOVIR VIATRIS or change the dosage without first checking with your doctor.
Do not let yourself run out of medicine over the weekend or on holidays.
Do not take any other medicines whether they require a prescription or not without first telling your doctor or consulting a pharmacist.
Be careful when handling VALGANCICLOVIR VIATRIS tablets. Do not break or crush them. If you accidentally touch broken or crushed tablets, wash your hands thoroughly with soap and water. If any powder from the tablet gets in your eyes, rinse your eyes thoroughly with water.
Be careful driving or operating machinery until you know how VALGANCICLOVIR VIATRIS affects you. VALGANCICLOVIR VIATRIS may cause drowsiness, dizziness, confusion or seizures (fits) in some people and therefore may affect alertness. If you have any of these symptoms, do not drive or operate machinery or do anything else that could be dangerous.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking VALGANCICLOVIR VIATRIS.
VALGANCICLOVIR VIATRIS helps most people with CMV infections but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. Your doctor has weighed the risks of using this medicine against the benefits they expect it will have for you.
If you are over 65 years of age you may have an increased chance of getting side effects.
Don't be alarmed by the list of possible side effects. You may not experience any of them
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
- dizziness
- looking pale
- diarrhoea
- nausea
- fever
- vomiting
- headache
These are the more common side effects of VALGANCICLOVIR VIATRIS and are usually short lived.
Tell your doctor immediately if you notice any of the following:
- skin rash
- abdominal pain
- cough
- fatigue (tiredness)
- oral thrush (sore, creamy yellow raised patches in the mouth)
- insomnia (inability to sleep)
- worsening of your eyesight
These side effects may be serious. You may require urgent medical attention.
Tell your doctor immediately, or go to Accident and Emergency at your nearest hospital, if you notice any of the following.
- swelling of the tongue, lips or throat
- any sign of infection such as fever, chills, sore throat or mouth ulcers
- unexplained bruising or bleeding
- thinking, hearing or seeing things that are not real
- confusion
- agitation
- fits
Tell your doctor or pharmacist if you notice anything else that is making you feel unwell, even if it is not on the list. This is not a complete list of all possible side effects. Others may occur in some people
Ask your doctor or pharmacist if you don't understand anything in this list.
After using VALGANCICLOVIR VIATRIS
Storage
Keep your tablets in the bottle until it is time to take them. If you take the tablets out of the bottle they may not keep well.
Keep VALGANCICLOVIR VIATRIS in a cool dry place where the temperature stays below 25°C.
Do not store it or any other medicine in a bathroom or near a sink. Do not leave it in the car or on a window sill. Heat and dampness can destroy some medicines.
Keep VALGANCICLOVIR VIATRIS where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking VALGANCICLOVIR VIATRIS, or the tablets have passed their expiry date, ask your pharmacist what to do with any tablets that are left over.
Product description
Availability
VALGANCICLOVIR VIATRIS comes in 450 mg film-coated tablets, in bottles containing 60 tablets.
What VALGANCICLOVIR VIATRIS looks like
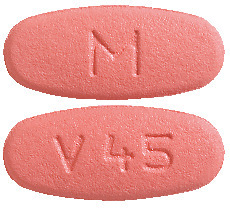
VALGANCICLOVIR VIATRIS - valganciclovir 450 mg are pink, filmcoated, oval, biconvex, beveled edge tablets debossed with M on one side of the tablet and V45 on the other side.
Ingredients
Active Ingredients - valganciclovir:
- each VALGANCICLOVIR VIATRIS tablet contains 450 mg of valganciclovir
Inactive Ingredients -
VALGANCICLOVIR VIATRIS film-coated tablets also contain
- Crospovidone
- Stearic acid
- Microcrystalline cellulose
- Hypromellose
- Titanium dioxide
- Macrogol 400
- Polysorbate 80
- Iron oxide red
VALGANCICLOVIR VIATRIS tablet contains trace amount of sulfites.
Supplier
VALGANCICLOVIR VIATRIS is supplied in Australia by:
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Australian registration numbers:
VALGANCICLOVIR VIATRIS: AUST R 218477
This leaflet was prepared in October 2023.
VALGANCICLOVIR VIATRIS_cmi\Oct23/00
Published by MIMS November 2023


 Refer to adult dosing for patients older than 16 years.
Refer to adult dosing for patients older than 16 years. Estimated creatinine clearance can be calculated from serum creatinine by the following formula:
Estimated creatinine clearance can be calculated from serum creatinine by the following formula:
 Based on two clinical trials (n = 370) where patients with CMV retinitis received valganciclovir at a dosage of 900 mg twice daily or once daily, corresponding to the induction or maintenance regimen, respectively, the adverse events with an incidence of ≥ 5%, regardless of seriousness and drug relationship is shown in Table 3. Approximately 65% of these patients received valganciclovir for more than nine months (maximum duration was 30 months).
Based on two clinical trials (n = 370) where patients with CMV retinitis received valganciclovir at a dosage of 900 mg twice daily or once daily, corresponding to the induction or maintenance regimen, respectively, the adverse events with an incidence of ≥ 5%, regardless of seriousness and drug relationship is shown in Table 3. Approximately 65% of these patients received valganciclovir for more than nine months (maximum duration was 30 months). Serious adverse events for valganciclovir from these three clinical trials (n = 934) with a frequency of less than 5% and which are not mentioned in Tables 2 and 3, are listed below:
Serious adverse events for valganciclovir from these three clinical trials (n = 934) with a frequency of less than 5% and which are not mentioned in Tables 2 and 3, are listed below:




 For study PV16000 a population pharmacokinetics analysis was conducted using plasma samples taken from 160/245 patients in the valganciclovir arm and 82/127 patients in the oral ganciclovir arm, and from this analysis it was estimated that the median exposure to ganciclovir from valganciclovir was 1.74 times higher than seen with oral ganciclovir (AUC0-24h 44.3 vs. 25.4 microgram.hour/mL).
For study PV16000 a population pharmacokinetics analysis was conducted using plasma samples taken from 160/245 patients in the valganciclovir arm and 82/127 patients in the oral ganciclovir arm, and from this analysis it was estimated that the median exposure to ganciclovir from valganciclovir was 1.74 times higher than seen with oral ganciclovir (AUC0-24h 44.3 vs. 25.4 microgram.hour/mL). The graft survival rate at 12 months post-transplant was 98.2% (160/163) for the 100-day dosing regimen and 98.1% (152/155) for the 200-day dosing regimen. The incidence of biopsy proven acute rejection at 12 months post-transplant was 17.2% (28/163) for the 100-day dosing regimen and 11.0% (17/155) for the 200-day dosing regimen.
The graft survival rate at 12 months post-transplant was 98.2% (160/163) for the 100-day dosing regimen and 98.1% (152/155) for the 200-day dosing regimen. The incidence of biopsy proven acute rejection at 12 months post-transplant was 17.2% (28/163) for the 100-day dosing regimen and 11.0% (17/155) for the 200-day dosing regimen. Decreased renal function resulted in decreased clearance of ganciclovir from valganciclovir, and a corresponding increase in terminal half-life. Therefore, dosage adjustment is required for renally impaired patients (see Section 4.4 Special Warnings and Precautions for Use; Section 4.2 Dose and Method of Administration).
Decreased renal function resulted in decreased clearance of ganciclovir from valganciclovir, and a corresponding increase in terminal half-life. Therefore, dosage adjustment is required for renally impaired patients (see Section 4.4 Special Warnings and Precautions for Use; Section 4.2 Dose and Method of Administration).

