What is in this leaflet
This leaflet answers some common questions about VERZENIO. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you taking VERZENIO against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What VERZENIO is used for
VERZENIO is an anticancer medicine containing the active substance abemaciclib.
VERZENIO is used to treat patients with certain types of breast cancer (hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-)) which have spread beyond the original tumour and/or to other organs. It is given together with aromatase inhibitors or fulvestrant, which are hormonal anticancer therapies (endocrine therapies).
VERZENIO belongs to a group of medicines called antineoplastic drugs. VERZENIO works by blocking proteins called cyclindependent kinase 4 and 6, that are abnormally active in some cancer cells and make them grow out of control. Continuously blocking these proteins can slow down the growth of cancer cells, shrink the tumour and delay the progression of your cancer.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
It is available only with a doctor's prescription.
There is not enough information to recommend the use of this medicine for children under the age of 18 years.
Before you take VERZENIO
When you must not take it
Do not take VERZENIO if you have an allergy to:
- abemaciclib
- any of the ingredients listed at the end of this leaflet.
VERZENIO contains lactose (a type of sugar found in milk or dairy products). If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
VERZENIO may decrease fertility in men.
Do not take this medicine if you are pregnant. It may affect your developing baby if you take it during pregnancy.
Do not breast-feed if you are taking this medicine. It is not known if VERZENIO is excreted into breast milk and if the breast-fed infant is at risk of harm. Talk to your doctor about breast-feeding during or after treatment with VERZENIO.
Do not give this medicine to a child or adolescent under the age of 18 years. Safety and effectiveness in children younger than 18 years have not been established.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start taking this medicine, talk to your doctor.
Before you start to take it
Your doctor will perform a blood test before and during treatment to check whether VERZENIO affects the number of white cells in your blood or the concentration in your blood of enzymes that come from your liver. VERZENIO may reduce the number of white blood cells and produce abnormalities in liver blood tests.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you have or have had any of the following medical conditions:
- severe liver problems
- blood clots in the legs or lungs
Tell your doctor if you are pregnant or plan to become pregnant or are breast-feeding. Your doctor can discuss with you the risks and benefits involved.
If you have not told your doctor about any of the above, tell him/her before you start taking VERZENIO.
Taking other medicines
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and VERZENIO may interfere with each other. These include:
- Clarithromycin
(antibiotic used to treat bacterial infections) - Ketoconazole, itraconazole
(both used to treat fungal infections) - Diltiazem
(used to treat chest pain (angina) and high blood pressure) - Verapamil
(used to treat chest pain (angina), high blood pressure and heart rhythm problems). - Rifampicin,
used to treat tuberculosis (TB), as it may reduce the effectiveness of VERZENIO.
These medicines may be affected by VERZENIO or may affect how well it works. You may need different amounts of your medicines, or you may need to take different medicines.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
How to take VERZENIO
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions provided, ask your doctor or pharmacist for help.
How much to take
When given together with aromatase inhibitors or fulvestrant to treat your breast cancer, the recommended dose of VERZENIO is 150 mg orally, twice daily.
How to take it
Swallow the tablet whole with a full glass of water.
Do not chew, crush or split the tablets before swallowing. VERZENIO may be taken with or without food.
Women treated with the combination of VERZENIO plus endocrine therapy should be in a postmenopausal state prior to therapy.
When to take it
Take your medicine at about the same time each day. Taking it at the same time each day will have the best effect. It ensures that there is enough VERZENIO in your body all the time. It will also help you remember when to take it.
It does not matter if you take this medicine before or after food, just avoid grapefruit products.
How long to take it
Treatment should be continued until disease progression or unacceptable toxicity. Consult your doctor regarding adverse events.
If you forget to take it
If you miss (or vomit) a dose, take your next dose at its scheduled time.
Do not take a double dose to make up for the dose that you missed.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Contact your doctor. You may get some of the side effects described below under Side effects.
While you are using VERZENIO
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking VERZENIO.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you are going to have surgery, tell the surgeon or anaesthetist that you are taking this medicine.
It may affect other medicines used during surgery.
If you become pregnant while taking this medicine, tell your doctor immediately.
If you are about to have any blood tests, tell your doctor that you are taking this medicine.
It may interfere with the results of some tests.
It is important to keep all of your doctor's appointments so that your progress can be checked.
Your doctor may do some tests from time to time to make sure the medicine is working and to prevent unwanted side effects.
There might be a support group of patients and their families who have the same disease as you. Ask your doctor or nurse if there is one that could help you.
Things you must not do
Do not take VERZENIO to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop taking your medicine or lower the dosage without checking with your doctor.
If necessary, your doctor will gradually reduce the amount you take each day before stopping the medicine completely.
Do not take grapefruit products while on this therapy as it may increase the side effects of VERZENIO.
Things to be careful of
VERZENIO has no known effect on the ability to drive and use machines. If you experience any symptoms affecting your ability to concentrate and react, do not drive or use machines until the effect goes away.
This medicine may cause fatigue and dizziness in some people. If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Be careful when drinking alcohol while you are taking this medicine.
If you feel light-headed, dizzy or faint when getting out of bed or standing up, get up slowly.
Standing up slowly, especially when you get up from bed or chairs, will help your body get used to the change in position and blood pressure. If this problem continues or gets worse, talk to your doctor.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking VERZENIO.
This medicine helps people with breast cancer, but it may have unwanted side effects as well. All medicines can have side effects. They may or may not be serious. You may need medical attention if you get some of the side effects. Your doctor may lower your dose or stop treatment temporarily to try to reduce certain side effects while you are taking VERZENIO.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
Ask your doctor or pharmacist to answer any questions you may have.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
- diarrhoea – it is a very common side effect of VERZENIO. At the first sign of diarrhoea, start treatment with antidiarrhoeal agents, such as loperamide. Drink plenty of fluids. If the diarrhoea does not resolve tell your doctor, he/she may lower your dose of VERZENIO or stop treatment temporarily.
- inflammation of the mouth and lips (stomatitis), nausea, vomiting, decreased appetite, dry mouth, alteration in taste
- dizziness, feeling of tiredness, muscular weakness
- infections, rash, itching, hair loss
The above list includes the more common side effects of your medicine.
Tell your doctor as soon as possible if you notice any of the following:
- fever or chills which could be a sign of low white blood cell counts (neutropenia). This is very common when taking VERZENIO. Contact your doctor immediately.
- blood clot in your veins
- dry skin
- increased tearing
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
If you have cough, fever and difficulty breathing or chest discomfort, this could be a sign of lung inflammation (pneumonitis). Serious or life-threatening infections are uncommon. Other side effects not listed above may also occur in some people.
Some of the side effects e.g. reduction in white blood cells, red blood cells, blood platelets and abnormalities in liver blood tests can only be found when your doctor does tests from time to time to check your progress.
These side effects may not be fully attributable to VERZENIO alone but may be caused by your condition or by other medicines that you may be taking at the same time.
After using VERZENIO
Storage
Keep your tablets in the pack until it is time to take them. If you take the tablets out of the pack they may not keep well.
Keep your tablets in a cool dry place where the temperature stays below 30°C.
Do not store VERZENIO or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop taking this medicine or the expiry date has passed, ask your pharmacist what to do with any medicine that is left over.
Product description
What it looks like
50 mg tablets: Beige, modified oval tablet debossed with "Lilly" on one side and "50" on the other.
100 mg tablets: white modified oval tablet debossed with "Lilly" on one side and "100" on the other.
150 mg tablets: yellow modified oval tablet debossed with "Lilly" on one side and "150" on the other.
The medicines may be available in pack sizes of 14, 42, 56 or 70 tablets.
(Not all pack sizes may be marketed.)
Ingredients
VERZENIO contains 50, 100 or 150 mg of abemaciclib as the active ingredient. Excipients used in the tablet are:
- croscarmellose sodium
- lactose monohydrate
- microcrystalline cellulose
- silicon dioxide
- sodium stearyl fumarate
- polyvinyl alcohol (E1203)
- titanium dioxide (E171)
- macrogol 3350 (E1521)
- purified talc (E553b)
- iron oxide yellow (E172) [50 mg and 150 mg tablets only]
- iron oxide red (E172) [50 mg tablets only]
This medicine does not contain sucrose, gluten, tartrazine or any other azo dyes.
Supplier
VERZENIO is supplied in Australia by:
Eli Lilly Australia Pty Limited
112 Wharf Road
WEST RYDE, NSW 2114
™ = Trademark
This leaflet was prepared in March 2020.
AUST R numbers:
304765: VERZENIO 50 mg
304767: VERZENIO 100 mg
304766: VERZENIO 150 mg
Published by MIMS May 2020



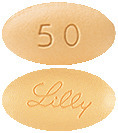







 With concomitant use of moderate CYP3A inhibitors, monitor for adverse reactions and consider reducing the Verzenio dose in 50 mg decrements as demonstrated in Table 1, if necessary.
With concomitant use of moderate CYP3A inhibitors, monitor for adverse reactions and consider reducing the Verzenio dose in 50 mg decrements as demonstrated in Table 1, if necessary.

 In a subsequent analysis (01 April 2021 data cut off), the median follow-up duration was 27 months in both arms, and 90% of patients were off treatment, including 72% who had completed the 2-year study treatment period. In the ITT population, Verzenio plus endocrine therapy reduced the hazard of developing an IDFS event by 30.4% (HR = 0.696, 95% CI [0.588, 0.823], nominal p ≤ 0.0001) compared to endocrine therapy alone and there was a 5.4% absolute improvement in the 3-year IDFS rate. In addition, the clinically meaningful benefit in DRFS (HR = 0.687, 95% CI [0.571, 0.826], nominal p ≤ 0.0001) was maintained with Verzenio plus endocrine therapy.
In a subsequent analysis (01 April 2021 data cut off), the median follow-up duration was 27 months in both arms, and 90% of patients were off treatment, including 72% who had completed the 2-year study treatment period. In the ITT population, Verzenio plus endocrine therapy reduced the hazard of developing an IDFS event by 30.4% (HR = 0.696, 95% CI [0.588, 0.823], nominal p ≤ 0.0001) compared to endocrine therapy alone and there was a 5.4% absolute improvement in the 3-year IDFS rate. In addition, the clinically meaningful benefit in DRFS (HR = 0.687, 95% CI [0.571, 0.826], nominal p ≤ 0.0001) was maintained with Verzenio plus endocrine therapy.

 Progression-free survival (PFS) was significantly prolonged in the Verzenio plus aromatase inhibitor (AI) arm, (hazard ratio [HR] of 0.540 [95% CI, 0.418 to 0.698]); median PFS was 28.18 months in the Verzenio plus AI arm and was 14.76 months in the placebo plus AI arm. These results correspond to a clinically meaningful reduction in the risk of disease progression or death of 46% for patients treated with abemaciclib plus an aromatase inhibitor.
Progression-free survival (PFS) was significantly prolonged in the Verzenio plus aromatase inhibitor (AI) arm, (hazard ratio [HR] of 0.540 [95% CI, 0.418 to 0.698]); median PFS was 28.18 months in the Verzenio plus AI arm and was 14.76 months in the placebo plus AI arm. These results correspond to a clinically meaningful reduction in the risk of disease progression or death of 46% for patients treated with abemaciclib plus an aromatase inhibitor.
 Median PFS was significantly prolonged in the Verzenio plus fulvestrant arm (HR of 0.553 [95% CI 0.449, 0.681]); median PFS was 16.4 months versus 9.3 months in the placebo plus fulvestrant arm. These results correspond to a clinically meaningful reduction in the risk of disease progression or death of 44.7% and a 7.2 month improvement in median PFS for patients treated with Verzenio plus fulvestrant. Early and sustained separation by treatment arm was apparent beginning at 8 weeks. Verzenio plus fulvestrant prolonged progression free survival with neither a clinically meaningful or significant detriment to health related quality of life.
Median PFS was significantly prolonged in the Verzenio plus fulvestrant arm (HR of 0.553 [95% CI 0.449, 0.681]); median PFS was 16.4 months versus 9.3 months in the placebo plus fulvestrant arm. These results correspond to a clinically meaningful reduction in the risk of disease progression or death of 44.7% and a 7.2 month improvement in median PFS for patients treated with Verzenio plus fulvestrant. Early and sustained separation by treatment arm was apparent beginning at 8 weeks. Verzenio plus fulvestrant prolonged progression free survival with neither a clinically meaningful or significant detriment to health related quality of life.
 Analyses for OS by stratification factors showed OS HR of 0.675 (95% CI: 0.511, 0.891) in patients with visceral disease, and 0.686 (95% CI: 0.451, 1.043) in patients with primary endocrine resistance.
Analyses for OS by stratification factors showed OS HR of 0.675 (95% CI: 0.511, 0.891) in patients with visceral disease, and 0.686 (95% CI: 0.451, 1.043) in patients with primary endocrine resistance. At the time of the final analysis of survival (minimum of 18 months follow-up), 19 of the 26 responding patients had responses of 6 months or longer, and 6 patients were still on treatment with response durations ranging from 9.5+ to 20.5+ months.
At the time of the final analysis of survival (minimum of 18 months follow-up), 19 of the 26 responding patients had responses of 6 months or longer, and 6 patients were still on treatment with response durations ranging from 9.5+ to 20.5+ months.
