What is in this leaflet
Read this leaflet carefully before you start taking this medicine.
This leaflet answers some common questions about ZALDIAR Film Coated Tablets.
It does not contain all of the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking ZALDIAR Film Coated Tablets against the benefits they expect it will have for you.
If you have any concerns about taking this medicine, talk to your doctor or pharmacist.
Keep this leaflet with your medicine. You may need to read it again.
What ZALDIAR is used for
ZALDIAR Film Coated Tablets contain a combination of two analgesics, tramadol hydrochloride and paracetamol, which act together to relieve your pain.
ZALDIAR is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
Ask your doctor if you have any questions about why ZALDIAR has been prescribed for you. Your doctor may have prescribed it for another reason.
ZALDIAR is available only with a doctor's prescription.
Before you take ZALDIAR
When you must not take it
Do not take ZALDIAR if you have an allergy (hypersensitivity) to:
- any medicine containing tramadol hydrochloride or paracetamol
- any of the ingredients listed at the end of this leaflet
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
- drop of blood pressure and fainting
Do not take ZALDIAR if you:
- have consumed a lot of alcohol
- have taken more than the recommended amount of:
- sleeping tablets
- other medication to relieve pain that your doctor has prescribed or that you have bought from a pharmacy, supermarket or health food shop
- psychotropic medications (medicines that affect mood and emotions) - are also taking or have taken MAO inhibitors within the last 14 days
- suffer from severe liver disorder
- have uncontrolled epilepsy
- weigh less than 37.5kg
Do not give ZALDIAR to children of 12 years and under. Safety and effectiveness in children younger than 12 years has not been established.
Do not take ZALDIAR if the expiry date (Exp.) printed on the pack has passed.
Do not take ZALDIAR if the packaging is torn or shows signs of tampering. If it has expired or the packaging is damaged, return it to your pharmacist for disposal.
Before you start to take Zaldiar
Tell your doctor if you are allergic to any other medicines, foods, dyes or preservatives.
Tell your doctor if you take other medicines containing paracetamol or tramadol hydrochloride.
Tell your doctor if you have, or have had, any medical conditions, especially the following:
- liver problems or liver disease or if you notice your eyes and skin turning yellow. This may suggest jaundice or problems with your bile ducts.
- kidney problems
- severe breathing difficulties, such as asthma or have severe lung problems
- epilepsy or have already experienced fits or seizures
- recent head injury, shock or severe headaches associated with vomiting
- any drug dependence
- taking other medicines to treat pain that contain buprenorphine or pentazocine
- dehydration
- eating disorders (anorexia, bulimia)
- a wasting syndrome including unexplained weight loss, fatique, weakness and loss of appetite
- malnutrition (low reserves of glutathione)
- hypovolaemia (decreased blood volume)
If you plan to have any surgery or procedure that requires an anaesthetic tell your doctor or dentist that you are taking ZALDIAR.
Tell your doctor if you drink alcohol.
Tell your doctor if you cannot have lactose. Zaldiar film coated tablets contain a small amount of lactose.
Tell your doctor if you are pregnant or plan to become pregnant or are breast feeding. This medicine contains tramadol hydrochloride which should not be taken during pregnancy and breast feeding.
If you become pregnant during treatment, consult your doctor before taking any further tablet.
If you have not told your doctor about any of the above, tell him/her before you start taking ZALDIAR.
Taking other medicines
Tell your doctor if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop
Tell your doctor if you are taking any other medicines which contain tramadol hydrochloride or paracetamol. This will ensure you do not exceed the maximum daily doses allowed for tramadol hydrochloride and / or paracetamol.
You must not take Zaldiar together with monoamine oxidase inhibitors (“MAOIs”)(see also section “Do not take Zaldiar”).
Some medicines may be affected by ZALDIAR Film Coated Tablets, or may affect how well it works.
Therefore, Zaldiar is not recommended to be taken with the following:
- Carbamazepine, a drug used to treat epilepsy or some types of pain
- Buprenorphine or pentazocine which are also used to treat pain
- Gabapentin or Pregabalin which are used to treat epilepsy or pain due to nerve problems (neuropathic pain)
The risk of side effects increases
- if you are taking triptans (for migraine) or selective serotonin re-uptake inhibitors, “SSRIs” (for depression). If you experience confusion, restlessness, fever, sweating, uncoordinated movement of limbs or eyes, uncontrollable jerking of muscles or diarrhoea you should call your doctor.
- if you are taking certain antidepressants. Zaldiar may interact with these medicines and you may experience symptoms such as involuntary, rhythmic contractions of muscles, including the muscles that control movement of the eye, agitation, excessive sweating, tremor, exaggeration of reflexes, increased muscle tension, body temperature above 38 °C.
- if you are taking tranquillizers, sleeping pills, other pain relievers such as morphine and codeine (also as cough medicine), baclofen (a muscle relaxant) medicines used to lower blood pressure, medicines to treat allergies. You may feel drowsy or feel faint. If this happens, tell your doctor.
- if you are taking medicines which may cause convulsions (fits), such as certain antidepressants, antipsychotics, bupropion (used to stop smoking). The risk of having a fit may increase if you take Zaldiar at the same time.
- if you are taking warfarin or phenprocoumon (for blood thinning). The effectiveness of such medicines may be altered and bleeding may occur. Any prolonged or unexpected bleeding should be reported to your doctor immediately.
The effect of Zaldiar may be altered if you also take:
- Metoclopramide, domperidone or ondansetron, used to treat nausea and vomiting
- Cholestyramine, used to reduce cholesterol in the blood
- Ketoconazole or erythromycin, used to treat infections
Your doctor will tell you whether Zaldiar is suitable for you.
If you are not sure whether you are taking any of these medicines, check with your doctor or pharmacist. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking ZALDIAR.
How to take ZALDIAR
Follow all directions given to you by your doctor or pharmacist carefully.
If you do not understand the instructions in this leaflet, ask your doctor or pharmacist for help.
How much to take
The usual starting dose for adults and adolescents over 12 years is 2 tablets.
Additional tablets can be taken as recommended by your doctor. The shortest time between doses must be at least 6 hours.
Do not take more than 8 tablets per day.
If you weigh between 37.5kg and 50kg you should not take more than 6 ZALDIAR tablets daily.
The dosage should be adjusted to the intensity of your pain and your individual pain sensitivity. In general the lowest pain-relieving dose should be taken.
Your doctor may reduce the maximum number of tablets you can take each day if you:
- Are over 75 years of age
- Have mild to moderate liver problems
- Have kidney problems
Your doctor will check how well ZALDIAR is working at regular intervals.
How to take ZALDIAR Film Coated Tablet
Swallow the tablet(s) with a full glass of water or other liquid.
Do not break or chew the tablet.
If you forget to take ZALDIAR
The pain is likely to return.
Take it as soon as you remember, and then go back to taking it as before.
Do not take a double dose to make up for the dose you missed.
If you are not sure what to do, ask your doctor or pharmacist.
How long to take ZALDIAR for
Withdrawal
Continue taking your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning
- increased sweating.
Zaldiar given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
If you take too much ZALDIAR (overdose)
If you or someone else receive too much (overdose), and experience one or more of the symptoms below, immediately call triple zero (000) for an ambulance. Keep the person awake by talking to them or gently shaking them every now and then. You should follow the above steps even if someone other than you have accidentally used Zaldiar that was prescribed for you. If someone takes an overdose they may experience one or more of the following symptoms:
- Slow, unusual or difficult breathing
- Drowsiness, dizziness or unconsciousness
- Slow or weak heartbeat
- Nausea or vomiting
- Convulsions or fits
If you think you or someone else may have used too much Zaldiar, you should immediately:
- phone the Poisons Information Centre (by calling 13 11 26), or
- contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
When seeking medical attention, take this leaflet and remaining medicine with you to show the doctor. Also tell them about any other medicines or alcohol which have been taken.
While you are taking ZALDIAR
Things you must do
Before starting any new medicine, tell your doctor or pharmacist that you are taking ZALDIAR.
If you become pregnant while taking ZALDIAR tell your doctor.
Keep all your doctor’s appointments so that your progress can be checked.
Tell your doctor if you feel the dose you are taking is too strong or too weak. If the dose is too strong you may feel very drowsy or have difficulty breathing.
If the dose is too weak you may continue to feel some pain between doses.
Things you must not do
Do not stop taking ZALDIAR, or change the dose, without checking with your doctor. On rare occasions, especially if you have been taking Zaldiar for a while stopping treatment abruptly can make you feel unwell.
Do not take ZALDIAR to treat any other conditions unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Be careful driving or operating machinery until you know how ZALDIAR affects you. It may cause dizziness and drowsiness in some people. Make sure you know how you react to Zaldiar before you drive a car, or do anything else that could be dangerous if you are dizzy.
This effect may be made worse by drinking alcohol or some other medicines that act on the central nervous system.
Addiction
You can become addicted to Zaldiar even if you take it exactly as prescribed. Zaldiar may become habit forming causing mental and physical dependence. If abused it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking Zaldiar. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking Zaldiar suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to Zaldiar may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking ZALDIAR.
This medicine helps most people with your condition, but it may have unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by this list of possible side effects. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
Very common:
- Nausea
- Dizziness
- Drowsiness
Common:
- Vomiting
- Digestion problems (constipation, wind, diarrhoea)
- Stomach pain
- Dry mouth
- Itching
- Sweating (hyperhidrosis)
- Headache
- Shaking
- Confusional state
- Sleep disturbances
- Mood change
Uncommon:
- Increase in pulse or blood pressure, heart rate or rhythm disorders
- Difficulty or pain on passing water
- Skin reactions (e.g. rash, hives)
- Tingling, numbness or feeling of pins and needles in the limbs, ringing in the ear, involuntary muscle twitching
- Depression, nightmares, hallucinations, memory lapses
- Difficulty swallowing
- Blood in stools
- Shivering, hot flushes, pain in chest
- Difficulty breathing
Rare:
- Fits, difficulties in carrying out coordinated movements
- Addiction
- Blurred vision
- Fainting (syncope)
- Speech disorder
- Delirium
- Constriction of the pupil (miosis)
- Excessive dilation of the pupils (mydriasis)
The following are recognized side effects which have been reported by people using medicines that contain only tramadol hydrochloride or only paracetamol.
However, tell your doctor as soon as possible if you notice any of the following:
- Feeling faint when getting up from a lying or sitting position
- Slow heart rate
- Fainting
- Changes in appetite
- Muscle weakness
- Slower or weaker breathing
- Mood changes
- Changes in activity
- Changes in perception
- Worsening of existing asthma
If any of the following happen, stop treatment and tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
- skin rash
- sudden swelling of the face and neck
- difficulties breathing
- drop of blood pressure and fainting
- yellowing of the skin and /or eyes, also called jaundice
Some of the above could be a sign of an allergic reaction. You may need urgent medical attention or hospitalisation. Allergic reactions are rare.
In rare cases, taking a medicine of the type of tramadol hydrochloride may make you become dependent on it, making it hard to stop taking it.
On rare occasions, people who have been taking tramadol hydrochloride for some time may feel unwell if they stop treatment abruptly. Symptoms may include:
- feeling agitated, anxious, nervous or shaky
Very few people may also get:
- panic attacks,
- hallucinations,
- unusual perceptions, such as itching, tingling and numbness, noise in the ears (tinnitus)
Other side effects not listed above may also occur in some patients. Tell your doctor if you notice anything that is making you feel unwell.
After taking ZALDIAR
Storage
Keep ZALDIAR where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Keep your medicine in a cool dry place where the temperature stays below 25°C. Do not refrigerate or freeze.
Keep your tablets in their packaging until it is time to take them. If you take them out of the carton box, and blister or foil pack, they may not keep well.
Do not store ZALDIAR or any other medicine in the bathroom or near a sink.
Do not leave ZALDIAR in the car or on window sills. Heat and dampness can destroy some medicines.
Disposal
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
Product description
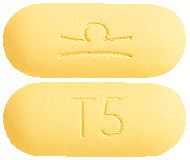
What it looks like
ZALDIAR Film Coated Tablets are pale yellow film-coated tablets, marked with the manufacturer‘s logo  on one side and marked ‘T5’ on the other side.
on one side and marked ‘T5’ on the other side.
The tablets are packed in a white opaque plastic blister pack
Each carton contains 2, 6, 10, 20 or 50 tablets, respectively.
Not all pack sizes may be marketed.
Ingredients
The active substances are tramadol hydrochloride and paracetamol.
Each tablet contains 37.5 mg of tramadol hydrochloride and 325 mg of paracetamol.
The other ingredients in the film coated tablet are:
Tablet core: powdered cellulose; pregelatinised maize starch; sodium starch glycollate (type A); maize starch; magnesium stearate.
Film-coating light yellow: hypromellose, lactose monohydrate, titanium dioxide, Macrogol 6000, iron oxide yellow, propylene glycol, purified talc.
Sponsor
Aspen Pharmacare, 34-36 Chandos St.,
St Leonards NSW 2065
Australian registration numbers:
Zaldiar Film Coated Tablets:
AUST R 179677
Date of preparation:
November 2024
Published by MIMS November 2025

 The safety profile of Zaldiar is characterized by the following adverse reactions. The most commonly adverse reactions were nausea, dizziness and somnolence, observed in more than 10% of the patients. All adverse reactions identified for Zaldiar are listed below under the corresponding body organ systems according to the following classification: very common ≥ 10%; common ≥ 1% to < 10%; uncommon ≥ 0.1% to < 1%; rare ≥ 0.01% to < 0.1%.
The safety profile of Zaldiar is characterized by the following adverse reactions. The most commonly adverse reactions were nausea, dizziness and somnolence, observed in more than 10% of the patients. All adverse reactions identified for Zaldiar are listed below under the corresponding body organ systems according to the following classification: very common ≥ 10%; common ≥ 1% to < 10%; uncommon ≥ 0.1% to < 1%; rare ≥ 0.01% to < 0.1%.
 Molecular formula: C16H25NO2.HCl. Relative molecular mass: Mr = 299.8.
Molecular formula: C16H25NO2.HCl. Relative molecular mass: Mr = 299.8. Molecular formula: C8H9NO2. Relative molecular mass: Mr = 151.2.
Molecular formula: C8H9NO2. Relative molecular mass: Mr = 151.2.