What is in this leaflet
This leaflet answers some common questions about Zoton FasTabs. It does not contain all the available information.
It does not take the place of talking to your doctor or pharmacist.
All medicines have benefits and risks. Your doctor has weighed the risks of you taking Zoton FasTabs against the benefits this medicine is expected to have.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
What Zoton FasTabs is used for
Peptic Ulcers
Zoton FasTabs is used to treat peptic ulcers in adults. Depending on the position of the ulcer it is either a gastric or duodenal ulcer. A gastric ulcer occurs in the stomach. A duodenal ulcer occurs in the duodenum, which is the tube leading out of the stomach.
Too much acid being made in the stomach can cause these ulcers.
Zoton FasTabs is also used to help stop duodenal ulcers from coming back.
Reflux Oesophagitis
Zoton FasTabs is used to treat the symptoms of reflux oesophagitis or reflux disease in adults and in children from 6 to 17 years of age. This can be caused by backflow (reflux) of food and acid from the stomach into the food pipe or gullet, also known as the oesophagus.
Reflux can cause a burning sensation in the chest rising up to the throat, also known as heartburn.
Heartburn and stomach pain associated with reflux or peptic ulcer.
Zoton FasTabs is used for the short-term treatment of heartburn and peptic ulcer symptoms in adults.
Peptic Ulcers Associated with Helicobacter Pylori Infection
Most people who have a peptic ulcer also have bacteria called Helicobacter pylori in their stomach. Zoton FasTabs can be taken in conjunction with certain antibiotics to help eradicate Helicobacter pylori and let your peptic ulcer heal. However, it is possible that the antibiotics may not always get rid of Helicobacter pylori.
How Zoton FasTabs works
Zoton FasTabs contains lansoprazole, which is a type of medicine called a proton pump inhibitor (PPI). It works by decreasing the amount of acid the stomach makes, to give relief from the symptoms of excessive acid and allow healing to take place. This does not stop food being digested in the normal way.
Ask your doctor if you have any questions about why Zoton FasTabs has been prescribed for you.
Your doctor may prescribe this medicine for another reason.
There is no evidence that Zoton FasTabs is habit-forming. This medicine is available only with a doctor's prescription.
Before you take Zoton FasTabs
When you must not take it
Do not take Zoton FasTabs if you have an allergy to:
- Lansoprazole
- Any medicines containing a proton-pump inhibitor
- Any of the ingredients listed at the end of this leaflet.
Some of the symptoms of an allergic reaction include:
- rash, itching, or hives;
- shortness of breath, wheezing or difficulty in breathing;
- swelling of the face, lips, tongue or other parts of the body.
Do not take Zoton FasTabs if you have severe liver disease.
Do not take Zoton FasTabs if you are already taking the medicine atazanavir. Atazanavir is used to treat HIV infection. If it is taken at the same time as Zoton FasTabs, it will not be absorbed properly and will be less effective in treating HIV infection.
Do not take Zoton FasTabs after the use by (expiry) date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should take this medicine, talk to your doctor.
Before you start to take it
You must tell your doctor if:
You have any allergies to any other medicines, foods, dyes or preservatives.
You are pregnant, plan to become pregnant or are breast-feeding.
Your doctor will discuss the possible risks and benefits of taking Zoton FasTabs during pregnancy.
Taking Zoton FasTabs during breast-feeding should be avoided as it is not known if this medicine passes into your breast milk.
You have any other medical conditions, including:
- Liver or kidney problems
- Inflammation of the bowel
- A tumour in the stomach region.
You have problems with digestion, or have an intolerance to:
- Fructose
- Glucose
- Galactose
- Lactose
- Sucrose.
If you have not told your doctor about any of the above, tell him or her before you take Zoton FasTabs.
Taking other medicines
Tell your doctor if you are taking any other medicines, including medicines that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with Zoton FasTabs. These medicines include:
- Theophylline used to treat asthma
- Oral contraceptives
- Warfarin used to prevent blood clots
- Carbamazepine and phenytoin used to treat seizures
- Ketoconazole used to treat fungal infections
- Digoxin used to treat heart complaints
- Sucralfate (used to treat gastric ulcers) and antacids (used to treat heartburn and indigestion)
Zoton FasTabs should be taken at least one hour before taking sucralfate or an antacid. - Iron preparations
- Ampicillin esters used in some antibiotics
- Tacrolimus used in transplant patients to reduce organ rejection
- Atazanavir, nelfinavir or other medicines used to treat HIV infection
- Methotrexate used to treat some cancers.
These medicines may be affected by Zoton FasTabs, or may affect how well it works. You may need different amounts of your medicine, or you may need to take different medicines. Your doctor will advise you.
How to take Zoton FasTabs
Follow all directions given to you by your doctor or pharmacist carefully. These may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
When to take it
Take Zoton FasTabs in the morning before food. Zoton FasTabs works best when taken on an empty stomach.
How much to take
Take one tablet each day, unless your doctor has told you otherwise.
Adults
The dose is usually 30 mg a day. The dose may vary from 15 mg to 30 mg a day depending on your condition.
Children (6 years or older)
For children between 6 to 11 years, the recommended dose depends on the weight of the child.
For children weighing 30 kg or less, the usual dose is 15 mg daily.
For children weighing over 30 kg, the usual dose is one 30 mg tablet daily.
For children between 12 to 17 years, the dose may vary from 15 mg to 30 mg a day depending on the condition.
How to take it
Swallow the tablet whole with a glass of water, or gently suck the tablet, then swallow the granules with your saliva. Do not chew or crush the tablet as this affects how well Zoton FasTabs works.
How long to take it
Keep taking Zoton FasTabs as directed, unless your doctor gives you other instructions.
In most patients, Zoton FasTabs relieves symptoms rapidly and healing is usually complete within 4 weeks. In some patients a further 4 weeks of treatment may be needed for complete healing.
In some cases, your doctor may decide that long-term treatment is needed.
Tell your doctor if any of your symptoms return after stopping long-term treatment.
Zoton FasTabs is recommended only for short-term use (8 to 12 weeks) in children.
For children aged 6-11 years, do not exceed 12 weeks of treatment with Zoton FasTabs.
For children aged 12-17 years, do not exceed 8 weeks of treatment with Zoton FasTabs.
Tell your doctor if your symptoms return. You may need further treatment.
If you forget to take it
If it is almost time for the next dose, skip the missed dose and take the next dose when you are meant to. Otherwise, take it as soon as you remember, and then go back to your normal routine.
Do not take a double dose to make up for the dose you missed.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much (overdose)
Immediately telephone your doctor or Poisons Information Centre (Tel 13 11 26) for advice, or go to Accident and Emergency at your nearest hospital, if you think that you or anyone else may have taken too much Zoton FasTabs. Do this even if there are no signs of discomfort or poisoning.
While you are taking Zoton FasTabs
Things you must do
Take Zoton FasTabs exactly as your doctor has prescribed.
Tell your doctor if you become pregnant while you are taking Zoton FasTabs.
If you are about to start any new medicine, remind your doctor and pharmacist that you are taking Zoton FasTabs.
Things you must not do
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not take Zoton FasTabs to treat any other complaints unless your doctor tells you to.
Do not stop taking your medicine or change the dosage without checking with your doctor. If you stop taking it suddenly, your condition may worsen or you may have unwanted side effects.
Things to be careful of
Be careful driving or operating machinery until you know how Zoton FasTabs affects you. Zoton FasTabs generally does not cause any problems with your ability to drive a car or operate machinery. However, as with many other medicines, Zoton FasTabs may cause dizziness in some people. Make sure you know how you react to Zoton FasTabs before you drive a car, operate machinery, or do anything else that could be dangerous if you are dizzy. If you drink alcohol, dizziness may be worse.
Things that may help your condition
Some self-help measures suggested below may help your condition. Talk to your doctor or pharmacist about these measures and for more information.
- Alcohol
Your doctor may advise you to limit your alcohol intake. - Aspirin and many other medicines used to treat arthritis, period pain or headaches
These medicines may irritate the stomach and may make your condition worse. Your doctor or pharmacist may suggest other medicines you can take. - Caffeine
Your doctor may advise you to limit the number of drinks that contain caffeine, such as coffee, tea, cocoa and cola drinks, because they contain ingredients that may irritate the stomach. - Eating habits
Eat smaller, more frequent meals. Eat slowly and chew your food carefully. Try not to rush at meal times. Eat your meals well before bedtime. - Smoking
Your doctor may advise you to stop smoking or at least cut down. - Weight
Your doctor may suggest losing some weight to help your condition.
Side effects
Tell your doctor as soon as possible if you do not feel well while taking Zoton FasTabs.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Do not be alarmed by the list of side effects. You may not experience any of them.
Ask your doctor or pharmacist any questions you may have.
Tell your doctor if you notice any of the following and they worry you:
Stomach or bowel problems such as:
- Vomiting or nausea
- Diarrhoea or constipation
- Stomach pain
- Indigestion
- Flatulence or wind.
If you suffer from severe persistent diarrhoea and/or vomiting when taking Zoton FasTabs, tell your doctor.
As natural acid in the stomach helps to kill bacteria, the lowering of acid by acid-reducing medicines such as Zoton FasTabs may cause some people to get certain stomach infections.
Difficulty thinking or working because of:
- Headache
- Dizziness
- Tiredness
- Joint or muscle aches or pains
- Generally feeling unwell
- Feeling confused, depressed or having hallucinations.
Changes to your appearance such as:
- Skin rashes
- Hives or itchy skin
- Hair thinning
- Breast enlargement and impotence in men with long term use.
Signs of infection such as:
- Coughs, colds, sore throats or sinuses indicating an upper respiratory tract infection
- Frequent and painful passing of urine indicating a urinary tract infection
- Dry or sore mouth or throat.
Changes in your sight, hearing, taste or touch such as:
- Tingling or numbness of hands and feet
- Blurred vision
- Increased sensitivity to sunlight
- Taste disturbances.
Tell your doctor immediately or go to Accident and Emergency at your nearest hospital if you notice any of the following:
- Red, itchy blistering spots, especially if it appears in areas of the skin that are exposed to the sun and is accompanied by joint pain
- Yellowing of the skin or eyes, especially if accompanied by fever, fatigue, loss of appetite, dark coloured urine or light coloured bowel movements
- Watery and severe diarrhoea
- Pain in the kidney region
- Swelling of the face, lips, tongue or throat, which may cause difficulty breathing
- Swelling of hands, ankles or feet
- Bruising or bleeding more easily than normal, bleeding under the skin or red or purple flat pinhead spots under the skin
- Severe blisters and bleeding in the lips, eyes, mouth, nose or genitals (Stevens-Johnson syndrome)
- Sudden or severe hives or itchy skin which may be accompanied by fever
- Frequent infections such as fever, severe chills, sore throat or mouth ulcers
- Cramping of the muscles in your hands or feet
- Irregular heartbeat
- Fits or seizures.
These are serious to very serious side effects. You may need urgent medical attention. These side effects are rare.
Your doctor may want to take some blood tests from time to time to check your progress.
Tell your doctor if you notice anything making you feel unwell when taking, or soon after finishing taking, Zoton FasTabs.
Other side effects not listed above may occur in some patients.
Other problems are more likely to arise from the ulcer itself rather than the treatment.
For this reason, contact your doctor immediately if you notice any of the following:
- Pain or indigestion occurring during treatment with Zoton FasTabs
- You begin to vomit blood or food
- You pass black (blood-stained) motions.
Ask your doctor or pharmacist if you do not understand anything in this list.
After taking Zoton FasTabs
Storage
Keep your tablets in their blister pack until it is time to take them. If you take the tablets out of the blister pack they may not keep well.
Keep it in a cool dry place where the temperature stays below 25°C. Do not store it or any other medicines in a bathroom or near a sink. Do not leave it in the car or on windowsills. Heat and dampness can destroy some medicines.
Keep it where young children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop Zoton FasTabs or the tablets have passed their expiry date, ask your pharmacist what to do with any tablets that are left over.
Product description
What it looks like
Zoton FasTabs 15 mg is available in a blister pack of 28 tablets.
Zoton FasTabs 30 mg is available in a blister pack of 7 or 28 tablets.
Zoton FasTabs 15 mg tablets are white to yellowish white uncoated tablets with orange to dark brown speckles, with "15" marked on one side.
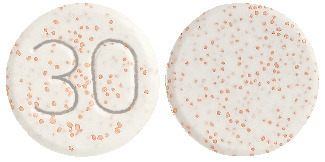
Zoton FasTabs 30 mg tablets are white to yellowish white uncoated tablets with orange to dark brown speckles, with "30" marked on one side.
Ingredients
Zoton FasTabs contain either 15 mg or 30 mg of lansoprazole as the active ingredient.
Zoton FasTabs also contain the inactive ingredients:
- Lactose monohydrate
- Microcrystalline cellulose
- Magnesium carbonate hydrate
- Low-substituted hyprolose
- Hyprolose
- Hypromellose
- Titanium dioxide
- Purified talc
- Mannitol
- Methacrylic acid - ethyl acrylate copolymer (1:1) 30 percent
- Polyacrylate dispersion 30 percent
- Macrogol 8000
- Citric acid
- Glyceryl monostearate
- Polysorbate 80
- Triethyl citrate
- Iron oxide yellow (E172)
- Iron oxide red (E172)
- Crospovidone
- Magnesium stearate
- Strawberry flavour
- Aspartame.
Zoton FasTabs do not contain gluten, tartrazine or any other azo dyes.
Australian Registration Number
Zoton FasTabs 15 mg tablets: AUST R 153575.
Zoton FasTabs 30 mg tablets: AUST R 153701.
Supplier
Pfizer Australia Pty Ltd
Sydney NSW
Toll Free Number: 1800 675 229.
www.pfizermedinfo.com.au
This leaflet was prepared in January 2023
© Pfizer Australia Pty Ltd 2019.
® Manufactured by and licensed from Takeda Chemical Industries Limited, Osaka Japan. Proprietor of the trademark Zoton FasTabs®.
Published by MIMS March 2023




 Treatment with lansoprazole also demonstrated significant reduction in frequency and severity of GORD symptoms (p < 0.001).
Treatment with lansoprazole also demonstrated significant reduction in frequency and severity of GORD symptoms (p < 0.001).
 The maintenance studies discussed, using lansoprazole 15 mg and 30 mg, did not extend beyond 12 months.
The maintenance studies discussed, using lansoprazole 15 mg and 30 mg, did not extend beyond 12 months. There was also a significant difference in the usage of "rescue" antacid treatment in the two groups, with 67% of the lansoprazole group taking antacids in the first two weeks of treatment compared with 83% of the ranitidine group (p=0.001).
There was also a significant difference in the usage of "rescue" antacid treatment in the two groups, with 67% of the lansoprazole group taking antacids in the first two weeks of treatment compared with 83% of the ranitidine group (p=0.001).
 Chemical name: 2[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulphinyl]-1H-benzimidazole.
Chemical name: 2[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulphinyl]-1H-benzimidazole.