MedicineInsight application process
MedicineInsight collects and uses data on the prescribing behaviour of general practitioners in Australia.
How do I get access to MedicineInsight data or reports?
An overview of the process for accessing MedicineInsight data or reports is shown below. A member of the Client Relations team will work with you to facilitate access to the most appropriate MedicineInsight data products in a timely fashion.
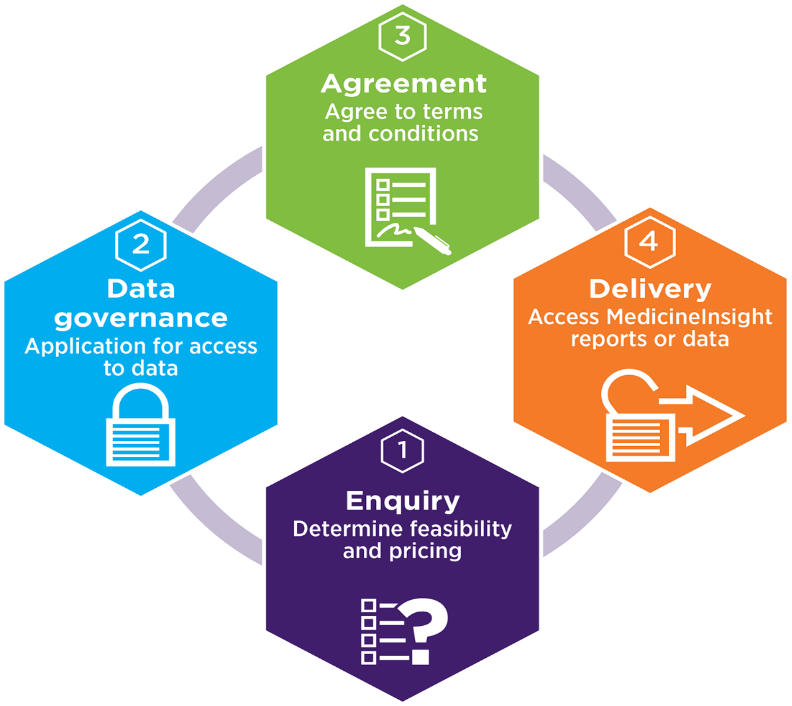
The first step to getting access to MedicineInsight data is to complete the Data Access Enquiry Form, available in the Research Kit. This form will help us understand your project and whether the data available in MedicineInsight can potentially answer your research questions.
For help completing the form, please email [email protected]
How much will access to the MedicineInsight data cost?
The cost of access to MedicineInsight data or a report will vary depending on what you request. Once the Client Relations team has an understanding of your request and your budget, we will discuss the options available. We are also able to provide a formal proposal or quotation for submitting as part of an application for research funding.
Once feasibility and price are determined and agreed, the next step is the formal governance approval process for access to MedicineInsight data.
NPS MedicineWise Data Governance Committee
The Data Governance Committee is an external and independent Committee established by NPS MedicineWise to provide decisions on the use of data collected under the MedicineInsight program. The Committee is a core function of the robust and well regarded NPS MedicineWise Data Governance Framework1 and operates in accordance with the principles that reflect the mission, goals and values of the organisation, including commitment to:-
- keeping the consumer at the centre of all our work;
- collaboration, consultation, and responsiveness to feedback from stakeholders;
- independent, balanced, accurate and relevant information and education; using evidence-based interventions and building the evidence base where gaps exist.
Functions of the Committee are to provide advice on general data governance issues (e.g. privacy, security, and data linkage), recommendations on data access applications submitted for review and to make decisions on data access for projects applying to use MedicineInsight data. The terms of reference for the Data Governance Committee can be found below.
- A person with suitable experience, and an understanding of, general practice data;
- Clinical general practitioners, including a general practitioner from a practice that is contributing data to MedicineInsight;
- At least one consumer representative with experience in research, and an understanding of privacy and ethics;
- Researcher(s) with expertise in scientific methods, such as epidemiology, biostatistics and observational studies;
- Individuals with expertise in any one or more of the following: general practice research, data linkage, public health, health policy, research and data ethics, data privacy considerations, data security
All decisions agreed upon by the Committee are made in strict accordance with the program’s ethics approval granted by the Royal Australian College of General Practitioners (RACGP), National Research Ethics and Evaluation Committee (approval number 17-017) for the standard operation and use of MedicineInsight. Within this framework, Committee decisions consider: study feasibility; research outputs; scientific validity; ethical appropriateness; benefits and public interest; secondary uses2 of the data as agreed to by data custodians (i.e. participating general practices) per the practice agreement (and RACGP secondary use framework) together with possible risks arising from community perception (i.e. from consumers/patients and the general practice community).
Applicants (internal and external) are reminded that approval by the Data Governance Committee does not replace the need to consider ethical obligations and obtain appropriate ethics approval, where required, for their project.
A publicly accessible register of projects approved by the Data Governance Committee is maintained by NPS MedicineWise and is available for review.
Categories of Committee Membership
Committee members are appointed as individuals for their expertise rather than in a representative capacity from an organisation. At a minimum, membership includes:-
Representatives from NPS MedicineWise are also in attendance at routine meetings to provide guidance and secretariat support as appropriate.
Meetings are held up to six times per calendar year. In order to maintain quorum, more than half of Committee members must be present at every meeting. Applicants are advised that personal information related to a members name and position currently remains confidential and is unable to be released publicly.
To ensure continuity of knowledge, members are appointed for a period of two years with a succession plan submitted to the Chief Executive (or delegate) at the end of each calendar year.
