What is in this leaflet
This leaflet answers some common questions about Amlodipine Sandoz. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risk of you taking Amlodipine Sandoz against the benefits it is expected to have for you.
If you have any concerns about taking this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine. You may want to read it again.
What Amlodipine Sandoz is used for
What it does
The name of your medicine is Amlodipine Sandoz. It contains the active ingredient amlodipine besilate.
Amlodipine Sandoz is used to treat high blood pressure. Everyone has blood pressure. This pressure helps to circulate the blood all around your body. Your blood pressure may be different at different times of the day, depending on how busy or worried you are. You have hypertension (high blood pressure) when your blood pressure stays higher than is needed, even when you are calm and relaxed.
There are usually no symptoms of hypertension. The only way of knowing that you have hypertension is to have your blood pressure checked on a regular basis. If high blood pressure is not treated it can lead to serious health problems. You may feel fine and have no symptoms, but eventually hypertension can cause stroke, heart disease and kidney failure. Amlodipine Sandoz helps to lower your blood pressure.
Amlodipine Sandoz is also used to treat angina pectoris. Angina is caused by a shortage of the supply of oxygen to the heart and is characterised by a painful and uncomfortable feeling in the chest. This feeling often spreads to the arms or neck, and sometimes also to the shoulders and back.
Amlodipine Sandoz is not for the relief of a sudden attack of angina. If such an attack occurs, you should take other medication that your doctor will have given to you.
Your doctor may have prescribed Amlodipine Sandoz for another reason. Ask your doctor if you have any questions about why Amlodipine Sandoz was prescribed for you.
Amlodipine Sandoz is available only with a doctor's prescription.
How Amlodipine Sandoz works
Amlodipine Sandoz belongs to a group of medicines called calcium channel blockers or calcium ion antagonists. It works by relaxing the blood vessels in your body, making it easier for your heart to pump blood around the body. It also widens the blood vessels leading to your heart and so helps to increase the supply of oxygen-rich blood to the heart.
Calcium channel blockers do not change the amount of calcium in your blood or bones.
There is no evidence that Amlodipine Sandoz is addictive.
Before you take Amlodipine Sandoz
When you must not take Amlodipine Sandoz
Do not take Amlodipine Sandoz if:
- You are allergic to the active ingredient or any of the inactive ingredients mentioned at the end of this leaflet under Product Description.
- You ever had an allergic reaction to other medicines of this drug class. Examples of these medicines are felodipine, nifedipine, or lercanidipine. If you are not sure whether you have ever taken any of these medicines, check with your doctor or pharmacist.
- You are pregnant or breastfeeding, unless permitted by your doctor.
- It is past its expiry date or the packaging appears to have been tampered with.
Amlodipine Sandoz is not recommended for the use in children as the safety and efficacy in this age group have not been established.
Before you start to take Amlodipine Sandoz
Tell your doctor if you have allergies to:
- Any other medicines, especially if they are in the same drug class as Amlodipine Sandoz.
- Any other substances, including foods, preservatives or dyes.
Tell your doctor if you are pregnant or plan to become pregnant. This medicine may affect your developing baby if you take it during pregnancy. Your doctor can discuss with you the risks and benefits involved.
Do not breast-feed if you are taking this medicine. The active ingredient in Amlodipine Sandoz passes into breast milk. Your baby may be affected.
Tell your doctor if you have or have had any medical conditions, especially the following:
- heart problems, including heart failure
- liver problems.
Taking other medicines
Tell your doctor if you are taking any other medicine, including any that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may be affected by Amlodipine Sandoz, or may affect how well it works. You may need to use different amounts of your medicine, or you may need to take different medicines.
Tell your doctor or pharmacist if you are taking any of the following:
- other medicines used to treat angina, such as diltiazem
- some antibiotics, such as erythromycin, clarithromycin or rifampicin
- some antifungals, such as ketoconazole or itraconazole
- anti-proteases, medicines used to treat HIV infection, such as ritonavir
- simvastatin, a medicine used to lower cholesterol
- ciclosporin, tacrolimus, sirolimus or everolimus, medicines used tosuppress the immune system
- temsirolimus, a medicine used to treat kidney cancer
- St John's Wort.
Your doctor or pharmacist has a complete list of medicines to be careful with or avoid while taking Amlodipine Sandoz.
If you have not told your doctor or pharmacist about any of the above, tell them before you start to take Amlodipine Sandoz.
How to take Amlodipine Sandoz
Follow all directions given to you by your doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box/bottle, ask your doctor or pharmacist for help.
How much to take
The usual dose of Amlodipine Sandoz is one 5 mg tablet each day. Your doctor may increase this to one 10 mg tablet each day.
Your doctor may prescribe another dose of Amlodipine Sandoz depending on your condition and how you respond to this medicine.
How to take it
Swallow the tablet with a full glass of water.
If you need to break Amlodipine Sandoz, hold tablet with both hands and snap along break line.

Take your tablets about the same time each day, either morning or evening. This will help you to get the best effect and also makes it easier to remember when to take it.
Amlodipine Sandoz can be taken with or without food.
How long to take it
You must take Amlodipine Sandoz every day.
Continue taking your medicine for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep taking your medicine even if you feel well.
If you forget to take your dose
If less than 12 hours have passed since the time when you should have taken your dose, then take your dose as soon as you remember, and continue to take it as you would normally.
Otherwise, skip the dose you missed and take your next dose when you are meant to.
Do not take a double dose to make up for the dose you missed. This may increase the chance of you getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering when to take your medicine, ask your pharmacist for some hints.
If you take too much
Immediately telephone your doctor, or the Poisons Information Centre (telephone 13 11 26) or go to Accident and Emergency at your nearest hospital, if you think you or anyone else has taken too much Amlodipine Sandoz.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much Amlodipine Sandoz, you may feel dizzy, light-headed or faint or have an irregular heart beat.
While you are using Amlodipine Sandoz
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking Amlodipine Sandoz.
Tell any other doctors, dentists, and pharmacists who treat you that you are taking this medicine.
If you become pregnant while taking this medicine, tell your doctor immediately.
Keep all of your doctor's appointments so that your progress can be checked. Your doctor may do some tests (add specific tests as per PI) from time to time to make sure the medicine is working and to prevent unwanted side effects.
Things you must not do
Do not take Amlodipine Sandoz to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Things to be careful of
Avoid eating large quantities of grapefruit or drinking large quantities of grapefruit juice. Grapefruit juice contains one or more components that alter the metabolism of some medicines, including Amlodipine Sandoz.
Drinking very large quantities (over 1.2 litres) of grapefruit juice each day while taking Amlodipine Sandoz may increase the effects of this medicine.
Be careful driving or operating machinery until you know how Amlodipine Sandoz affects you. Amlodipine Sandoz may cause dizziness or light-headedness in some people, especially after the first dose or after the dose has been increased.
If you have any of these symptoms, do not drive, operate machinery or do anything else that could be dangerous.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking Amlodipine Sandoz.
Amlodipine Sandoz helps most people but it may have some unwanted side effects in a few people. All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
It can be difficult to tell whether side effects are the result of taking Amlodipine Sandoz, effects of your condition or side effects of other medicines you may be taking. For this reason it is important to tell your doctor of any change in your condition.
Do not be alarmed by the list of possible side effects. You may not experience any of them.
Tell your doctor if you notice any of the following and they worry you:
- headache
- dizziness
- flushing
- tiredness
- drowsiness or sleepiness
- stomach pain or nausea.
These are the more common side effects of Amlodipine Sandoz. Mostly, these are mild and short-lived.
Tell your doctor if you notice any of the following:
- indigestion
- sexual problems.
These may or may not be due to Amlodipine Sandoz but you should tell your doctor if they worry you.
Tell your doctor immediately if you notice any of the following:
- changes in heart beat (fast or slow)
- swelling of the ankles, feet, face or hands
- tingling or numbness of the hands or feet
- dizziness or light-headedness on standing up from a sitting or lying position
- unusual tiredness or weakness
- muscle cramps or aches
- joint pain
- eye pain or change in vision
- changes in mood, feeling anxious or nervous
- symptoms of liver disease such as itching, yellowing of the skin and eyes, and dark coloured urine
- unusual movements, including trembling and shaking of the hands, and fingers, twisting movements of the body, shuffling walk and stiffness of the arms and legs.
These may be serious side effects of Amlodipine Sandoz. You may need urgent medical attention. Serious side effects are rare.
If any of the following happen, stop taking Amlodipine Sandoz and tell your doctor immediately, or go to Accident and Emergency at your nearest hospital:
- swelling of the face, lips, mouth or throat which may cause difficulty in swallowing or breathing.
- shortness of breath
- symptoms of allergy such as skin rash and/or itching
- severe upper stomach pain, often with nausea and vomiting
- fast or irregular heart beats
- chest pain
- chest pain associated with exertion (angina) that lasts longer, is more severe or occurs more often.
These are very serious side effects. You may need urgent medical attention or hospitalisation. All of these side effects are very rare.
If you are 65 years or older, you should be especially careful when you are taking Amlodipine Sandoz.
Some people in this age group are more likely to experience side effects such as swelling of the feet and ankles, muscle cramps and dizziness.
Make sure to tell your doctor if you experience any side effects.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Other side effects not listed above may also occur in some people.
After using Amlodipine Sandoz
Storage
Keep Amlodipine Sandoz in the original packaging until you need to take it.
Store below 25°C in a dry place, where it is protected from light. Keep your tablets where children cannot reach them. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Do not leave your medicines on a window sill or in the car. Heat and dampness can destroy some medicines.
Disposal
Return any unused or out of date medicine to your pharmacist.
Product description
What Amlodipine Sandoz looks like
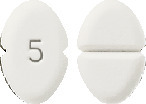
Amlodipine Sandoz 5mg: A white or almost white, oblong tablet, scored on one side and coded "5" on the other. Available in blisters of 30 tablets.
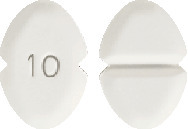
Amlodipine Sandoz 10mg: A white or almost white, oblong tablet, scored on one side and coded "10" on the other. Available in blisters of 30 tablets.
Ingredients
Active Ingredient
Each Amlodipine Sandoz 5mg tablet contains 5 mg amlodipine as amlodipine besilate.
Each Amlodipine Sandoz 10mg tablet contains 10 mg amlodipine as amlodipine besilate.
Inactive Ingredients
Each Amlodipine Sandoz tablet also contains sodium starch glycollate, calcium hydrogen phosphate, cellulose-microcrystalline and magnesium stearate.
Supplier
Sandoz Pty Ltd
ABN 60 075 449 553
54 Waterloo Road
Macquarie Park, NSW 2113
Australia
Tel: 1800 726 369
This leaflet was revised in September 2018.
Australian Register Numbers
Amlodipine Sandoz 5 mg tablets: AUST R 124578 (blisters)
Amlodipine Sandoz 10 mg tablets: AUST R 124582 (blisters)
Published by MIMS November 2018

 Other adverse experiences which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following (see Table 2).
Other adverse experiences which were not clearly dose related but which were reported with an incidence greater than 1.0% in placebo controlled clinical trials include the following (see Table 2). The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the doctor to a possible relationship.
The following events occurred in ≤ 1% but > 0.1% of patients in controlled clinical trials or under conditions of open trials or marketing experience where a causal relationship is uncertain; they are listed to alert the doctor to a possible relationship.
