SUMMARY CMI
APO-DIMETHYL FUMARATE
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I taking APO-DIMETHYL FUMARATE?
APO-DIMETHYL FUMARATE contains the active ingredient dimethyl fumarate. APO-DIMETHYL FUMARATE is used to treat relapsing multiple sclerosis (MS).
For more information, see Section 1. Why am I taking APO-DIMETHYL FUMARATE? in the full CMI.
2. What should I know before I take APO-DIMETHYL FUMARATE?
Do not use if you have ever had an allergic reaction to dimethyl fumarate or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I take APO-DIMETHYL FUMARATE? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with APO-DIMETHYL FUMARATE and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I take APO-DIMETHYL FUMARATE?
- The recommended starting dose of APO-DIMETHYL FUMARATE is 120 mg taken twice daily. After 7 days the recommended dose is 240 mg twice daily.
More instructions can be found in Section 4. How do I take APO-DIMETHYL FUMARATE? in the full CMI.
5. What should I know while taking APO-DIMETHYL FUMARATE?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while taking APO-DIMETHYL FUMARATE? in the full CMI.
6. Are there any side effects?
Common side effects are reddening of the face or body or body feeling warm, hot, burning or itchy (flushing), loose stools (diarrhoea), feeling sick (nausea), stomach pain or stomach cramps, inflammation of the lining of the intestines (gastroenteritis), being sick (vomiting), indigestion (dyspepsia), inflammation of the lining of the stomach (gastritis), gastrointestinal disorder, burning sensation, hot flush, feeling hot, itchy skin (pruritus), rash, pink or red blotches on the skin (erythema), runny nose (rhinorrhoea), hair thinning (alopecia). Serious side effects are signs of infection (e.g. unexplained fever, severe diarrhoea). For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
APO-DIMETHYL FUMARATE
Active ingredient: dimethyl fumarate
Consumer Medicine Information (CMI)
This leaflet provides important information about using APO-DIMETHYL FUMARATE. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using APO-DIMETHYL FUMARATE.
Where to find information in this leaflet:
1. Why am I taking APO-DIMETHYL FUMARATE?
2. What should I know before I take APO-DIMETHYL FUMARATE?
3. What if I am taking other medicines?
4. How do I take APO-DIMETHYL FUMARATE?
5. What should I know while taking APO-DIMETHYL FUMARATE?
6. Are there any side effects?
7. Product details
1. Why am I taking APO-DIMETHYL FUMARATE?
APO-DIMETHYL FUMARATE contains the active ingredient dimethyl fumarate.
APO-DIMETHYL FUMARATE is used to treat relapsing multiple sclerosis (MS).
APO-DIMETHYL FUMARATE slows down the progression of physical disability in people with relapsing forms of MS and decreases the number of flare ups (relapses).
Some people feel better when they start to take APO-DIMETHYL FUMARATE. However APO-DIMETHYL FUMARATE cannot repair damage that has already been caused by MS.
When you start APO-DIMETHYL FUMARATE you might not notice an improvement, but APO-DIMETHYL FUMARATE may still be working to help prevent your MS from becoming worse.
The cause of MS is not yet known, MS affects the brain and spinal cord. In MS, the body's immune system reacts against its own myelin (the 'insulation' surrounding nerve fibres). In relapsing forms of MS, people have 'exacerbations' from time to time (e.g. blurred vision, weakness in the legs or arms, or loss of control of bowel or bladder function). They are followed by periods of recovery. Recovery may be complete or incomplete. If it is incomplete there is 'progression of disability'.
Dimethyl fumarate decreases the inflammation in your brain that is caused by MS and thereby reduces nerve damage. It works by reducing inflammatory responses in cells and helps to protect the central nervous system cells against attack. Inflammation of the brain is an important part of the MS disease process.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
Dimethyl fumarate has not been studied in patients with chronic progressive MS.
2. What should I know before I take APO-DIMETHYL FUMARATE
Warnings
Do not use APO-DIMETHYL FUMARATE if:
- you are allergic to dimethyl fumarate, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips tongue or other parts of the body
- rash, itching or hives on the skin. - You are being treated with other medicines containing fumaric acid (creams or tablets/capsules).
Check with your doctor if you:
- have any other medical conditions such as
- liver problems
- kidney problems
- infection - recently received a vaccination
- take any other medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Shingles
Tell your doctor at the earliest opportunity if you suspect you may have shingles (a painful viral infection with a painful rash that develops on one side of the face or body).
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. There is no information on the use of APO-DIMETHYL FUMARATE during pregnancy. Your doctor will discuss the risks and benefits of taking it if you are pregnant.
Talk to your doctor if you are breastfeeding or intend to breastfeed. It is not known whether APO-DIMETHYL FUMARATE passes into breast milk. Your doctor will discuss the risks and benefits of taking it if you are breastfeeding or planning to breastfeed.
Children
Safety and effectiveness in children younger than 18 years have not been established.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines may interfere with APO-DIMETHYL FUMARATE and affect how it works. These include:
- medicines that contain fumaric acid (creams or tablets/capsules)
- medicines which affect the immune function including other medicines to treat MS such as fingolimod, natalizumab or mitoxantrone or some other commonly used cancer medicines
- medicines which affect the kidneys, including some antibiotics (used to treat infections), "water tablets" (diuretics), certain types of painkillers (such as ibuprofen and other similar anti-inflammatory medicines and medicines purchased without a doctor's prescription) and medicines that contain lithium
- live vaccines.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect APO-DIMETHYL FUMARATE.
4. How do I take APO-DIMETHYL FUMARATE?
How much to take
- The recommended starting dose of APO-DIMETHYL FUMARATE is 120 mg taken twice daily. After 7 days the recommended dose is 240 mg twice daily.
- Your doctor may tell you to take APO-DIMETHYL FUMARATE with aspirin or may temporarily reduce your dose.
Do not reduce your dose unless your doctor tells you to.
When to take APO-DIMETHYL FUMARATE
- Take one capsule twice a day. Taking it at the same time each day (e.g. in the morning during breakfast and at night during dinner) will help you remember when to take it.
- Continue taking your medicine for as long as your doctor tells you. This medicine helps to control your condition, but does not cure it. The positive effects of APO-DIMETHYL FUMARATE may not be seen immediately. It is important to keep taking your medicine even if you feel well.
- It is important not to interrupt treatment with APO-DIMETHYL FUMARATE unless your doctor tells you to.
How to take APO-DIMETHYL FUMARATE
- Swallow each capsule whole with a glass of water. Do not crush, divide or dissolve the capsule or its contents.
- APO-DIMETHYL FUMARATE can be taken with or without food. For those patients who experience gastrointestinal side effects or flushing, taking APO-DIMETHYL FUMARATE with food may help reduce these effects.
- Do not drink alcohol for 1 hour after taking this medicine, as alcohol may lead to gastrointestinal side effects.
If you forget to take APO-DIMETHYL FUMARATE
APO-DIMETHYL FUMARATE should be used regularly at the same time each day.
If you miss a dose, and it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Otherwise, take it as soon as you remember, and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for the dose you missed.
If you use too much APO-DIMETHYL FUMARATE
If you think that you have used too much APO-DIMETHYL FUMARATE, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while taking APO-DIMETHYL FUMARATE?
Things you should do
Take APO-DIMETHYL FUMARATE exactly as your doctor has prescribed.
Keep all of your doctor's appointments so that your progress can be checked.
If you are about to have any blood or urine tests, tell your doctor that you are taking APO-DIMETHYL FUMARATE. Blood and urine test results may be affected by treatment with APO-DIMETHYL FUMARATE.
Tell your doctor if you are going to be vaccinated.
If you are about to start on any new medicine, remind your doctor and pharmacist that you are taking APO-DIMETHYL FUMARATE.
Remind any doctor, dentist or pharmacist you visit that you are taking APO-DIMETHYL FUMARATE.
Tell your partner or caregiver about your treatment.
Call your doctor straight away if you:
- think you have an infection, have fever, or feel like you have the flu.
APO-DIMETHYL FUMARATE may decrease lymphocyte (white blood cell) counts. White blood cells fight infection. You may get infections more easily while you are taking APO-DIMETHYL FUMARATE. Any infection that you already have may get worse. Infections could be serious and sometimes life-threatening. If you have a serious infection, your doctor may recommend that you stop taking APO-DIMETHYL FUMARATE until you recover. - think you are experiencing symptoms similar to an MS relapse, new or worsening weakness on one side of the body, clumsiness, changes in vision, thinking, or memory, or confusion or personality changes lasting for more than several days.
These could be signs of a rare and very serious brain infection called progressive multifocal leukoencephalopathy (PML). The symptoms of PML may be similar to an MS relapse.
Having low lymphocyte levels, particularly for a long period of time can increase your risk of PML.
Things you should not do
- Do not take APO-DIMETHYL FUMARATE to treat any other complaints unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not stop taking your medicine or lower the dosage without checking with your doctor.
Blood and urine tests
- Before you start APO-DIMETHYL FUMARATE, your doctor will do a blood test to check the number of your white blood cells. Your doctor may also test these periodically during treatment.
- Before you start APO-DIMETHYL FUMARATE, your doctor will make sure you have results from a recent urine test to check your kidney function and may repeat the test periodically during treatment. APO-DIMETHYL FUMARATE may cause proteins (such as albumin) to be detected in a urine test.
- APO-DIMETHYL FUMARATE may also cause increases in the level of liver enzymes that will show up in a blood test.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how APO-DIMETHYL FUMARATE affects you.
Drinking alcohol
Tell your doctor if you drink alcohol.
Do not drink alcohol for 1 hour after taking this medicine, as alcohol may lead to gastrointestinal side effects.
Looking after your medicine
- Keep your capsules in the pack until it is time to take them. If you take the capsules out of the pack they may not keep well.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight, where the temperature stays below 25°C. For example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine, it shows signs of tampering or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away if you notice any of these serious side effects. |
Very serious side effects
| Serious side effects | What to do |
| Call your doctor straight away or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What APO-DIMETHYL FUMARATE contains
| Active ingredient (main ingredient) |
|
| Other ingredients (inactive ingredients) |
|
| Potential allergens |
|
Gluten free, lactose free.
Do not take this medicine if you are allergic to any of these ingredients.
What APO-DIMETHYL FUMARATE looks like
APO-DIMETHYL FUMARATE 120 mg
ARTG 308780
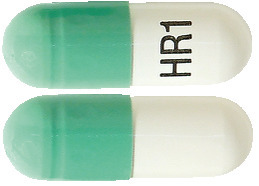
The 120 mg capsules are size “0” hard gelatin capsules with green cap and white body, printed with “HR1” in black ink on capsule body containing white to off-white, round, biconvex enteric coated mini tablets plain on both the sides.
Available in blisters containing 7, 14 or 112 capsules packed in a box.
APO-DIMETHYL FUMARATE 240 mg
ARTG 309012
The 240 mg capsules are size “0” hard gelatin capsules with green cap and body, printed with “HR2” in black ink on capsule body containing white to off-white, round, biconvex enteric coated mini tablets plain on both the sides.
Available in blisters containing 7, 14 or 56 capsules packed in a box.
Not all pack sizes may be available.
Further information
You can obtain more information from your doctor, pharmacist or the MS Society in your State, or by telephoning the MS Alliance on 1800 852 289 in Australia or 0800 852 289.
Who distributes APO-DIMETHYL FUMARATE
Arrotex Pharmaceuticals Pty Ltd
15-17 Chapel St, Cremorne VIC 3121
www.arrotex.com.au
This leaflet was prepared in November 2023.
Published by MIMS January 2024



 Other relevant ADRs (< 2% difference) include: gastroenteritis, gastritis, gastrointestinal disorder, burning sensation, feeling hot, alanine aminotransferase increased, proteinuria, white blood cell count decreased and leucopenia.
Other relevant ADRs (< 2% difference) include: gastroenteritis, gastritis, gastrointestinal disorder, burning sensation, feeling hot, alanine aminotransferase increased, proteinuria, white blood cell count decreased and leucopenia. Study 2 (CONFIRM) was a 2-year multicentre, randomised, double-blind, placebo-controlled study which contained a rater-blinded (i.e. study physician/investigator assessing the response to study treatment is blinded) reference comparator of glatiramer acetate (GA) in 1417 patients with RRMS.
Study 2 (CONFIRM) was a 2-year multicentre, randomised, double-blind, placebo-controlled study which contained a rater-blinded (i.e. study physician/investigator assessing the response to study treatment is blinded) reference comparator of glatiramer acetate (GA) in 1417 patients with RRMS. Pooled results at 2 years for Study 1 and Study 2 showed consistent and statistically significant results for dimethyl fumarate versus placebo in all primary and secondary endpoints, including time to confirmed disability progression (32% relative reduction compared to placebo).
Pooled results at 2 years for Study 1 and Study 2 showed consistent and statistically significant results for dimethyl fumarate versus placebo in all primary and secondary endpoints, including time to confirmed disability progression (32% relative reduction compared to placebo).

 DMF is a white to off-white powder that is slightly soluble in water. It has a molecular formula of C6H8O4 and a molecular weight of 144.13. The chemical name for DMF is dimethyl (2E)but-2-enedioate.
DMF is a white to off-white powder that is slightly soluble in water. It has a molecular formula of C6H8O4 and a molecular weight of 144.13. The chemical name for DMF is dimethyl (2E)but-2-enedioate.