SUMMARY CMI
IPTAM® MIGRAINE RELIEF
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using IPTAM MIGRAINE RELIEF?
IPTAM MIGRAINE RELIEF contains the active ingredient sumatriptan (as succinate). It is used to relieve a migraine headache in patients who have a stable, well-established pattern of symptoms. Sumatriptan should not be used to prevent migraine headaches from occurring. For more information, see Section 1. Why am I using IPTAM MIGRAINE RELIEF? in the full CMI.
2. What should I know before I use IPTAM MIGRAINE RELIEF?
Do not use if you have ever had an allergic reaction to sumatriptan succinate or any of the ingredients listed at the end of the CMI. Talk to your doctor or pharmacist if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding. For more information, see Section 2. What should I know before I use IPTAM MIGRAINE RELIEF? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with IPTAM MIGRAINE RELIEF and affect how it works. Tell your doctor or pharmacist if you are taking any other medicines, including any that you buy without a prescription from a pharmacy, supermarket or health food shop particularly herbal preparations containing St John's Wort. A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use IPTAM MIGRAINE RELIEF?
- The usual recommended dose for adults aged 18 to 65 years is 50 mg (one tablet).
- Take one tablet (50 mg) as soon as the migraine headache begins.
- If the first IPTAM MIGRAINE RELIEF tablet helps your migraine, but the migraine comes back later, you may take another tablet. Wait at least two hours after the first tablet was taken.
- Do NOT take more than 100 mg (2 tablets) in any twenty-four hour period, unless advised by your doctor.
More instructions can be found in Section 4. How do I use IPTAM MIGRAINE RELIEF? in the full CMI.
5. What should I know while using IPTAM MIGRAINE RELIEF?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using IPTAM MIGRAINE RELIEF? in the full CMI.
6. Are there any side effects?
Tell your doctor immediately and do not take any more tablets if you feel heaviness or tightness in any part of the body, irregular heartbeats, have wheezing, swelling of the lips/mouth, difficulty in breathing, hay fever, lumpy rash ("hives"), fainting, have a fit or convulsion or persistent purple discolouration and/or pain in response to cold. For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
IPTAM® MIGRAINE RELIEF
Active ingredient: sumatriptan (as succinate)
Consumer Medicine Information (CMI)
This leaflet provides important information about using IPTAM MIGRAINE RELIEF. Keep this leaflet with your medicine. You may need to read it again. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using IPTAM MIGRAINE RELIEF.
Where to find information in this leaflet:
1. Why am I using IPTAM MIGRAINE RELIEF?
2. What should I know before I use IPTAM MIGRAINE RELIEF?
3. What if I am taking other medicines?
4. How do I use IPTAM MIGRAINE RELIEF?
5. What should I know while using IPTAM MIGRAINE RELIEF?
6. Are there any side effects?
7. Product details
1. Why am I using IPTAM MIGRAINE RELIEF?
IPTAM MIGRAINE RELIEF contains the active ingredient sumatriptan succinate. IPTAM MIGRAINE RELIEF belongs to a group of medicines called serotonin agonists.
IPTAM MIGRAINE RELIEF is used to relieve a migraine headache in patients who have a stable, well-established pattern of symptoms. They should not be used to prevent migraine attacks from occurring. Do not use IPTAM MIGRAINE RELIEF unless your condition has been diagnosed by a doctor.
It is thought that migraine headache is due to widening of certain blood vessels in the head. These medicines work by making the blood vessels normal again and ease the symptoms of migraine.
Your IPTAM MIGRAINE RELIEF tablets do not work in other types of headaches which are not migraine.
IPTAM MIGRAINE RELIEF is not recommended for use in children and adolescents under the age of 18 as its safety and effectiveness has not been established in this group.
There is no evidence that IPTAM MIGRAINE RELIEF is addictive.
2. What should I know before I use IPTAM MIGRAINE RELIEF?
Warnings
Do not use IPTAM MIGRAINE RELIEF if:
- you have not been previously diagnosed with migraine by a doctor
- you are allergic to sumatriptan succinate, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine. - you have or have had:
- heart disease or heart attack
- shortness of breath, pain or tightness in the chest, jaw or upper arm
- peripheral vascular disease (pain in the back of the legs) or are prone to cold, tingling or numb hands and feet
- Prinzmetal's angina (an uncommon form of angina where pain is experienced at rest rather than during activity)
- angina
- high blood pressure
- stroke
- severe liver disease - you have taken any of these medicines in the last 24 hours:
- ergotamine (eg Cafegot)
- dihydroergotamine (eg Dihydergot)
- methysergide (eg Deseril)
- rizatriptan (eg Maxalt)
- eletriptan (eg Relpax)
- naratriptan (eg Naramig)
- zolmitriptan (eg Zomig) - you have taken any of these medicines in the last two weeks used for depression:
- monoamine oxidase inhibitors (MAOIs)
- selective serotonin reuptake inhibitors (SSRIs)
- serotonin noradrenaline reuptake inhibitors (SNRIs) - the packaging shows signs of tampering or the tablets do not look quite right
Check with your doctor or pharmacist if you:
- have allergies to foods, dyes, preservatives or any other medicines, including any that contain sulfonamide
- are allergic to lactose
- plan to have surgery
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Tell your doctor or pharmacist if you have or have had any of the following medical conditions:
- liver or kidney problems or any other medical conditions
- problems with your heart or blood vessels, or a family history of heart problems
- high blood pressure, even if it is under control
- high blood cholesterol levels
- obesity
- diabetes
- you are male and over 40 years of age
- you are female and have undergone menopause
- you smoke
- epilepsy, seizures or fits or been told that you are prone to this problem
- stroke
Tell your doctor or pharmacist if your migraine headache becomes more frequent. Overuse of this medicine may worsen your condition.
IPTAM MIGRAINE RELIEF may cause drowsiness. If affected, do not drive a vehicle or operate machinery.
Pregnancy and breastfeeding
Check with your doctor or pharmacist if you are pregnant or intend to become pregnant.
Your doctor or pharmacist will discuss the risks and benefits of taking IPTAM MIGRAINE RELIEF during pregnancy.
Talk to your doctor or pharmacist if you are breastfeeding or intend to breastfeed.
Your doctor or pharmacist will discuss the risks and benefits of taking IPTAM MIGRAINE RELIEF when breastfeeding.
Use in Children and Adolescents (under 18 years)
- Do not use in children or adolescents under the age of 18 years.
Use in the elderly (over 65 years)
- Not recommended for patient over the age of 65 years.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop particularly herbal preparations containing St John's Wort.
Consult your doctor or pharmacist before use, if you are taking other medicines including for migraine.
Consult your doctor or pharmacist, if you are taking medicine for depression, anxiety or the oral contraceptive pill. Some medicines may be affected by IPTAM MIGRAINE RELIEF or may affect how well it works.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect IPTAM MIGRAINE RELIEF.
4. How do I take IPTAM MIGRAINE RELIEF?
Follow all directions given to you by your doctor and pharmacist carefully.
How much to take
Adults 18 to 65 years
- The usual recommended dose for adult is 50 mg (one tablet). Take one tablet (50 mg) as soon as the migraine headache begins.
- If the first IPTAM MIGRAINE RELIEF tablet helps your migraine, but the migraine comes back later, you may take another IPTAM MIGRAINE RELIEF tablet. Wait at least two hours after the first tablet was taken.
- Do not take more than 100 mg (2 tablets) of IPTAM MIGRAINE RELIEF tablets in any 24-hour period.
- Do not take more IPTAM MIGRAINE RELIEF tablets, or any other form of IPTAM MIGRAINE RELIEF, if the first dose has not provided any relief from your symptoms.
- You may take your usual headache relief medication provided it does not contain ergotamine or methysergide.
If you are unsure what to do, ask your doctor or pharmacist. - If your migraine is not relieved by IPTAM MIGRAINE RELIEF, you may use IPTAM MIGRAINE RELIEF tablets on another occasion to treat another migraine headache.
- Follow the instructions on the pack.
- Do not exceed the recommended dosage.
When to take it
- IPTAM MIGRAINE RELIEF should be used:
(i) when the migraine headache begins; or
(ii) when other symptoms of the migraine begin, such as nausea (feeling sick), vomiting or your eyes becoming sensitive to light - If you take your tablet later during the migraine headache it will still work for you. Do not take your IPTAM MIGRAINE RELIEF tablet before the above symptoms occur.
How to take it
- Swallow the tablets whole with a glass of water. Do not crush or chew the tablet as it has a bitter taste.
If you use too much IPTAM MIGRAINE RELIEF
If you think that you have used too much IPTAM MIGRAINE RELIEF, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using IPTAM MIGRAINE RELIEF?
Things you should do
- Tell your doctor if, for some reason, you have not taken your medicine exactly as directed. Otherwise, your doctor may think that it is not working and change your treatment unnecessarily.
- Tell your doctor or pharmacist if your migraine headache lasts for longer than 24 hours.
- Tell your doctor or pharmacist if your pattern of symptoms has changed.
- Tell your doctor of pharmacist if you generally have four or more migraine headaches each month.
- Tell your doctor or pharmacist if you feel this headache is different or worse than your usual migraine e.g. more frequent, more persistent, more severe, or you don't recover between attacks.
- Tell your doctor or pharmacist if you have these symptoms with your migraine headache; weakness on one side of your body, double vision, clumsy and uncoordinated movements, tinnitus (ringing in the ears), reduced levels of consciousness, seizure like movements, recent rash with a headache.
Remind any doctor, dentist, pharmacist or other health professionals you visit that you are using IPTAM MIGRAINE RELIEF.
Things you should not do
- Do not give IPTAM MIGRAINE RELIEF to anyone else, even if their symptoms seem similar to yours
Medication overuse headaches
Overuse of migraine medications, such as IPTAM MIGRAINE RELIEF, have been associated with worsening of headaches (medication overuse headache or MOH) in some patients.
Overuse of medications for relief of headache and migraine can worsen your condition and lead to:
- Increase of headache frequency, and
- Can make your condition less responsive to treatments
If you are experiencing frequent or daily headaches, it is possible that you may be experiencing medication overuse headache. If your migraines have become more frequent, you should speak with your doctor or pharmacist.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how IPTAM MIGRAINE RELIEF affects you.
IPTAM MIGRAINE RELIEF may cause drowsiness, dizziness or light-headedness in some people (especially after the first dose). If any of these occur, do not drive, operate machinery or do anything else that could be dangerous.
If you use IPTAM MIGRAINE RELIEF tablets too often, it may make your headache worse. If this happens, your doctor may tell you to stop taking IPTAM MIGRAINE RELIEF tablets.
Drinking alcohol
Tell your doctor or pharmacist if you drink alcohol.
Looking after your medicine
- Keep your tablets in the pack until it is time to take them.
If you take the tablets out of the pack they will not keep well. - Keep your tablets in a place where the temperature stays below 25°C
Follow the instructions on the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor or pharmacist if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
| Call your doctor straight away and do not take any more IPTAM MIGRAINE RELIEF tablets if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available upon consultation with a pharmacist.
What IPTAM MIGRAINE RELIEF contains
| Active ingredient (main ingredient) | sumatriptan succinate |
| Other ingredients (inactive ingredients) |
|
| Potential allergens |
|
Do not take this medicine if you are allergic to any of these ingredients.
What IPTAM MIGRAINE RELIEF looks like
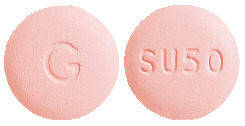
IPTAM MIGRAINE RELIEF is a pink, round, film-coated tablet with 'G' on one side and 'SU50' on the other side, supplied in blister packs of 2 tablets (AUST R 397730).
Who distributes IPTAM MIGRAINE RELIEF
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in October 2022.
IPTAM® is a Viatris Company trademark
IPTAM MIGRAINE RELIEF_cmi\Oct22/00
Published by MIMS April 2023



 Sumatriptan succinate is a white to off-white powder, with a melting point between 164.6°C-165.5°C, freely soluble in water, sparingly soluble in methanol and practically insoluble in methylene chloride.
Sumatriptan succinate is a white to off-white powder, with a melting point between 164.6°C-165.5°C, freely soluble in water, sparingly soluble in methanol and practically insoluble in methylene chloride.