SUMMARY CMI
Dolased Forte
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
WARNING: Important safety information is provided in a boxed warning in the full CMI. Read before using this medicine.
1. Why am I using Dolased Forte?
Dolased Forte contains the active ingredients paracetamol, codeine phosphate hemihydrate and doxylamine succinate. Dolased Forte is for the short-term management of severe pain where your doctor decides other treatment options are ineffective, are not recommended, not tolerated, or otherwise inappropriate to manage your pain.
For more information, see Section 1. Why am I using Dolased Forte? in the full CMI.
2. What should I know before I use Dolased Forte?
Do not use if you have ever had an allergic reaction to Dolased Forte or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use Dolased Forte? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with Dolased Forte and affect how it works.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use Dolased Forte?
The usual dose for adults and children between 12 and 18 years is one or two tablets every 4-6 hours as needed for relief.
More instructions can be found in Section 4. How do I use Dolased Forte? in the full CMI.
5. What should I know while using Dolased Forte?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Drinking alcohol |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using Dolased Forte? in the full CMI.
6. Are there any side effects?
Common side effects include skin rash, constipation, nausea, vomiting, dizziness, drowsiness, sweating, blurred vision. Serious side effects include, wheezing, difficulty breathing or shallow breathing, swelling of the face, lips, tongue or other parts of the body, rash, itching or hives.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
WARNING:
Limitations of use
Dolased Forte should only be used when your doctor decides that other treatment options are ineffective, not tolerated or otherwise inadequate to provide appropriate management your pain.
Hazardous and harmful use
Dolased Forte contains codeine which may be habit forming. Misuse, abuse, or addiction may lead to overdose and death. Your doctor will assess your risks and monitor your treatment regularly.
Life threatening respiratory depression
Serious, life-threatening or fatal respiratory depression (shallow or difficulty breathing) may occur with the use of Dolased Forte. Your doctor will assess your risks of respiratory depression and monitor your treatment regularly.
Concomitant use of benzodiazepines and other central nervous system (CNS) depressants, including alcohol
Using opioids while taking benzodiazepines (medicines used as sedatives or to treat anxiety), gabapentinoids (medicines used for epilepsy or neuropathic pain), antihistamines, antidepressants, antipsychotics, gabapentinoids (e.g. gabapentin and pregabalin), cannabis or other central nervous system (CNS) depressants, including alcohol, may result in extreme drowsiness and sleepiness, respiratory depression (shallow or difficulty breathing), coma, and death. Your doctor will limit your dosage and duration of use and monitor you regularly. Do not drink alcohol while taking Dolased Forte.
FULL CMI
Dolased Forte
Active ingredients: paracetamol, codeine phosphate and doxylamine succinate
Consumer Medicine Information (CMI)
This leaflet provides important information about using Dolased Forte. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using Dolased Forte.
Where to find information in this leaflet:
1. Why am I using Dolased Forte?
2. What should I know before I use Dolased Forte?
3. What if I am taking other medicines?
4. How do I use Dolased Forte?
5. What should I know while using Dolased Forte?
6. Are there any side effects?
7. Product details
1. Why am I using Dolased Forte?
Dolased Forte contains three active ingredients,
- paracetamol,
- codeine phosphate, and
- doxylamine succinate.
Paracetamol is an analgesic (pain-relieving medicine) and antipyretic (lowers body temperature). Codeine phosphate hemihydrate is a potent analgesic that helps to relieve pain. The body must convert codeine into morphine before it can provide pain relief. Doxylamine succinate is an antihistamine which can help to relieve tension and nausea associated with pain. This may be especially useful in the treatment of tension headache, migraine and period pain.
Dolased Forte is used for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain. This may include toothache and other dental pain, pain from injury or surgery, or headache pain.
It is suitable for people who cannot take aspirin for pain relief.
2. What should I know before I use Dolased Forte?
Warnings
Do not use Dolased Forte if:
- you are allergic to paracetamol, codeine phosphate, doxylamine succinate, or any of the ingredients listed at the end of this leaflet.
Always check the ingredients to make sure you can use this medicine.
- you have or have had any of the following medical conditions:
- severe and/or acute respiratory diseases, such as bronchitis, acute asthma, emphysema, acute chronic obstructive pulmonary disease (COPD).
- other problems with breathing such as, shallow breathing, difficulty breathing, or slow breathing.
- Glucose-6-phosphate-dehydrogenasedeficiency (an enzyme deficiency that may lead to anaemia)
- you very rapidly metabolise codeine into morphine.
- known intolerance to paracetamol.
- severe liver disease or liver failure - you have diarrhoea caused by antibiotics or poisoning.
- you are in the third trimester of pregnancy or in labour, especially if the baby is premature.
- you are breastfeeding or planning to breastfeed.
- you have a history of drug dependence, including alcohol dependence.
- you are already taking Monoamine Oxidase Inhibitors (MAOIs) such as selegiline or moclobemide, or within 14 days of stopping MAOIs.
- you have experienced allergy (generalised rash or shortness of breath) to morphine or oxycodone.
- the person going to take the tablets is under 12 years.
- you are aged between 12 and 18 years of age and have had your tonsils or adenoids removed to treat sleep apnoea.
Check with your doctor if you:
- have allergies to
- any other medicines
- aspirin or any other non-steroidal anti-inflammatory drugs (NSAIDs)
- any other substances, such as foods, preservatives or dyes - have or have had any medical conditions, especially the following:
- liver problems
- kidney problems
- Gilbert's syndrome
- heart problems
- low blood pressure
- lung problems, difficulty breathing, wheezing, chronic cough, asthma, or other chronic breathing conditions
- intolerance to pain relieving medicine
- chronic alcohol use, or if you recently stopped taking alcohol
- opioid dependence
- a history of drug and/or alcohol abuse.
Caution is particularly recommended for use in adolescents and young adults with a history of drug and/or alcohol abuse.
- low levels of glutathione
- recent stomach, intestine or urinary tract surgery
- gall bladder problems or your gall bladder has been removed
- obstructive and inflammatory bowel disease or other bowel problems
- chronic constipation
- multiple sclerosis
- prostate problems
- retaining urine
- problems passing urine
- underactive thyroid
- adrenal gland problems such as Addison's disease
- head injury
- brain tumour, stroke or other problems with the brain
- fits or seizures - take any medicines for any other condition
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
Do not take Dolased Forte during the third trimester of pregnancy.
Do not take Dolased Forte during labour, especially if the baby is premature.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
Do not take Dolased Forte if you are breastfeeding or planning to breastfeed.
Use in Children
Do not give Dolased Forte to children under 12 years.
Use in the Elderly
If you are over 65 years of age, talk to your doctor or pharmacist about how much to take.
Elderly patients are more likely to have less effective kidney function due to age. This may increase the risk of side effects.
Addiction
You can become addicted to Dolased Forte even if you take it exactly as prescribed. Dolased Forte may become habit forming causing mental and physical dependence. If abused it may become less able to reduce pain.
Dependence
As with all other opioid containing products, your body may become used to you taking Dolased Forte. Taking it may result in physical dependence. Physical dependence means that you may experience withdrawal symptoms if you stop taking Dolased Forte suddenly, so it is important to take it exactly as directed by your doctor.
Tolerance
Tolerance to Dolased Forte may develop, which means that the effect of the medicine may decrease. If this happens, more may be needed to maintain the same effect.
Withdrawal
Continue taking your medicine for as long as your doctor tells you. If you stop having this medicine suddenly, your pain may worsen and you may experience some or all of the following withdrawal symptoms:
- nervousness, restlessness, agitation, trouble sleeping or anxiety
- body aches, weakness or stomach cramps
- loss of appetite, nausea, vomiting or diarrhoea
- increased heart rate, breathing rate or pupil size
- watery eyes, runny nose, chills or yawning increased sweating.
Dolased Forte given to the mother during labour can cause breathing problems and signs of withdrawal in the newborn.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Tell your doctor or pharmacist if you are taking any other medicines, which contain paracetamol, codeine or doxylamine.
Do not take Dolased Forte with Monoamine Oxidase inhibitors (MAOIs), such as selegiline or moclobemide, or within 14 days of stopping MAOIs.
Some medicines may interfere with Dolased Forte including:
- medicines causing sleepiness or drowsiness, such as sedatives or tranquilisers
- Benzodiazepines (medicines used as sedatives or to treat anxiety). The use of opioids and benzodiazepines together may increase the risk of drowsiness, difficulties in breathing, coma and may be life-threatening.
- medicines containing alcohol (ethanol), such as some cough syrups
- cough suppressants or antitussives
- medicines used to treat alcohol and/or opioid dependence, such as naltrexone or buprenorphine
- medicines used to treat depression
- phenothiazines and antipsychotic agents (medicines used to treat mental disorders)
- some types of antihistamines
- aspirin or any other NSAIDs
- Gabapentinoids (medicines used for epilepsy or neuropathic pain)
- cannabis
- medicines used to stop or prevent vomiting (antiemetics), such as metoclopramide or domperidone
- medicines used to treat diarrhoea, such as kaolin or loperamide
- medicines used to thin the blood, such as warfarin
- propantheline (medicine used to treat stomach ulcers)
- medicines used to treat epilepsy or fits, such as phenytoin
- other pain relief medication
- medicines used to treat high blood pressure
- colestyramine (medicine used to treat bile problems or lower cholesterol)
- chelating resin
- chloramphenicol, an antibiotic used to treat ear and eye infections
- neuromuscular blocking agents such as cisatracurium (medicines used during surgical procedures)
- flucloxacillin, zidovudine or rifampicin (medicines used to treat infections)
Medicines that may reduce the effect of Dolased Forte include:
- medicines that inhibit the liver enzyme, CYP 2D6 inhibitors such as fluoxetine, paroxetine, bupropion, cinacalcet, and methadone
- medicines that increase the activity of the liver enzyme, CYP 3A4 inducers such as rifampicin.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect Dolased Forte.
4. How do I use Dolased Forte?
How much to take
- Adult and children between 12 and 18 years
- The usual adult dose is one or two tablets every 4 to 6 hours as needed for relief.
- Do not exceed 8 tablets in a 24 hour period.
- Dolased Forte must not be used in children under 12 years.
- Follow the instructions provided and use Dolased Forte until your doctor tells you to stop. Their directions may differ from the information contained in this leaflet.
How to take Dolased Forte
- Swallow the prescribed dose of Dolased Forte whole with a glass of water. It can be taken with or without food.
If you forget to take Dolased Forte
If you miss your dose at the usual time, take it as soon as you remember. If it is almost time for your next dose, skip the dose you missed and take your next dose when you are meant to.
Remember to wait 4 to 6 hours between doses.
Do not take a double dose to make up for the dose you missed.
If you take too much Dolased Forte
If you think that you have used too much Dolased Forte, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(by calling 13 11 26), or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using Dolased Forte?
Things you should do
- Remind any doctor including surgeons, dentist or pharmacist you visit that you are taking Dolased Forte.
- Keep all your appointments, including those for blood tests.
Call your doctor straight away if you:
- feel you need to take the medicine for longer periods of time
- feel you need to take more than the prescribed dose
- feel very unwell when you stop taking the medicine but feel better when you start taking the medicine again.
Things you should not do
- Do not stop using this medicine suddenly, or change the dose, without checking with your doctor.
- Do not take high doses of the medicine for long periods of time unless your doctor tells you to.
- Do not take Dolased Forte to treat any other conditions unless your doctor tells you to.
- Do not give your medicine to anyone else, even if they have the same condition as you.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how Dolased Forte affects you.
Dolased Forte may cause dizziness, drowsiness, affect eyesight, or hand-eye coordination in some people. Do not drive a car, operate machinery, or do anything else that could be dangerous while taking Dolased Forte.
Drinking alcohol
Tell your doctor if you drink alcohol.
Alcohol and codeine taken together may increase the risk of sedation, respiratory problems, coma or may be life-threatening.
Do not drink alcohol while you are taking Dolased Forte.
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Keep your tablets in the pack until it is time to take them.
Store it in a cool dry dark place where the temperature stays below 25°C, away from moisture, heat or sunlight; do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
Do not be alarmed by this list of possible side effects. You may not experience any of them. See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
Skin related:
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
Heart related:
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these very serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What Dolased Forte contains
| Active ingredient (main ingredient) | paracetamol 450 mg codeine phosphate 30 mg doxylamine succinate 5 mg |
| Other ingredients (inactive ingredients) | crospovidone glyceryl monostearate lactose monohydrate magnesium stearate pregelatinised maize starch povidone stearic acid |
Dolased Forte does not contain tartrazine or any other azo dyes.
Do not take this medicine if you are allergic to any of these ingredients.
What Dolased Forte looks like
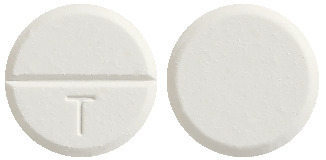
Dolased Forte is a round, white tablet with “T” on one side of a break line. Dolased Forte is available in a blister pack of 2, 20 or 40 tablets (AUST R 203987).
Not all pack sizes may be marketed.
Who distributes Dolased Forte
Aspen Pharmacare Australia Pty Ltd
34-36 Chandos St
St Leonards NSW 2065
Australia
www.aspenpharma.com.au
This leaflet was prepared in June 2023.
Published by MIMS August 2023