SUMMARY CMI
TEMIZOLE®
Consumer Medicine Information (CMI) summary
The full CMI on the next page has more details. If you are worried about using this medicine, speak to your doctor or pharmacist.
1. Why am I using TEMIZOLE?
TEMIZOLE contains the active ingredient temozolomide. TEMIZOLE is used to treat patients with brain tumours. TEMIZOLE is also used to treat adult patients with advanced metastatic malignant melanoma.
For more information, see Section 1. Why am I using TEMIZOLE? in the full CMI.
2. What should I know before I use TEMIZOLE?
Do not use if you have ever had an allergic reaction to TEMIZOLE or any of the ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines, or are pregnant or plan to become pregnant or are breastfeeding.
For more information, see Section 2. What should I know before I use TEMIZOLE? in the full CMI.
3. What if I am taking other medicines?
Some medicines may interfere with TEMIZOLE and affect how it works. Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
A list of these medicines is in Section 3. What if I am taking other medicines? in the full CMI.
4. How do I use TEMIZOLE?
- Your doctor has worked out the exact dose of TEMIZOLE for you according to your individual needs.
- Each time you start a new treatment cycle, be sure you understand exactly how many capsules of each strength you need to take on each day of dosing.
More instructions can be found in Section 4. How do I use TEMIZOLE? in the full CMI.
5. What should I know while using TEMIZOLE?
| Things you should do |
|
| Things you should not do |
|
| Driving or using machines |
|
| Looking after your medicine |
|
For more information, see Section 5. What should I know while using TEMIZOLE? in the full CMI.
6. Are there any side effects?
Like all medicines, TEMIZOLE may have unwanted side effects. Sometimes they are serious, most of the time they are not. Your doctor will discuss these with you and will explain the risks and benefits of using TEMIZOLE.
For more information, including what to do if you have any side effects, see Section 6. Are there any side effects? in the full CMI.
FULL CMI
TEMIZOLE®
Active ingredient(s): temozolomide
Consumer Medicine Information (CMI)
This leaflet provides important information about using TEMIZOLE. You should also speak to your doctor or pharmacist if you would like further information or if you have any concerns or questions about using TEMIZOLE.
Where to find information in this leaflet:
1. Why am I using TEMIZOLE?
2. What should I know before I use TEMIZOLE?
3. What if I am taking other medicines?
4. How do I use TEMIZOLE?
5. What should I know while using TEMIZOLE?
6. Are there any side effects?
7. Product details
1. Why am I using TEMIZOLE?
TEMIZOLE contains the active ingredient temozolomide. TEMIZOLE belongs to a group of medicines called cytotoxic or chemotherapy medicines.
TEMIZOLE works by killing cancer cells and stopping cancer cells from growing and multiplying.
TEMIZOLE is used to is used to treat patients with brain tumours. TEMIZOLE is also used to treat adult patients with advanced metastatic malignant melanoma.
Your doctor may have prescribed it for another reason.
Use in children
TEMIZOLE can be used to treat children aged 3 years and older, with specific forms of brain cancer (glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after standard therapy).
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
2. What should I know before I use TEMIZOLE?
Warnings
Do not use TEMIZOLE if:
- you are allergic to temozolomide, dacarbazine or any of the ingredients listed at the end of this leaflet.
- you or your partner are pregnant or intend to become pregnant
- you are breastfeeding
- you have very low levels of white blood cells, red blood cells or platelets
- Always check the ingredients to make sure you can use this medicine.
Symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin.
Check with your doctor if you:
- vomit frequently
- Your doctor may give you other medication to control the vomiting. - are anaemic or have blood clotting problems
- intend to have children
- TEMIZOLE may cause infertility in men. - have liver or kidney problems
- TEMIZOLE could cause hepatitis B to become active again, which can be fatal in some cases. - have allergies to any other medicines, foods, preservatives or dyes
- have any other medical conditions
- take any medicines for any other condition.
During treatment, you may be at risk of developing certain side effects. It is important you understand these risks and how to monitor for them. See additional information under Section 6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant.
TEMIZOLE may cause birth defects if either the male or female is taking this medicine at the time of conception or during pregnancy. Therefore, female patients must have a negative pregnancy test before starting TEMIZOLE. Both male and female patients and their partners should each use birth control whilst taking TEMIZOLE. Female patients should continue to use an effective form of birth control for at least 6 months and male patients to continue for at least 3 months after the final dose. Male patients whose partners are already pregnant should use a condom to minimise exposure to the unborn baby by TEMIZOLE in the sperm. Also, do not donate sperm during and for at least 3 months after the final dose due to the potential effects on sperm.
Breastfeeding
- Talk to your doctor if you are breastfeeding or intend to breastfeed.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any medicines, vitamins or supplements that you buy without a prescription from your pharmacy, supermarket or health food shop.
Some medicines and TEMIZOLE may interfere with each other. These include other medicines used to treat cancer or any other treatments that may affect your immune system. You may need different amounts of your medicines or you may need to take different medicines. Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins or supplements you are taking and if these affect TEMIZOLE.
4. How do I use TEMIZOLE?
How much to take
- Your doctor has worked out the exact dose of TEMIZOLE for you according to your individual needs.
- You may be given other medication to take before or after TEMIZOLE to help stop nausea.
TEMIZOLE in combination treatment with radiation (newly diagnosed patients)
- If you are a patient with a newly diagnosed brain tumour, your doctor will start you on a dose of TEMIZOLE every day for 42 days (up to 49 days) in combination with radiation therapy. This is the first part of the treatment ("concomitant phase") in which you complete the radiation therapy. Your treatment will be interrupted for 4 weeks to give your body a chance to recover.
- You will then start the next phase of treatment ("adjuvant phase") and your TEMIZOLE dose will change. In this phase, there are up to 6 treatment cycles. Each treatment cycle lasts 28 days. You will take your new dose of TEMIZOLE once a day for the first five days ("dosing days") of each cycle, followed by 23 days without TEMIZOLE. This makes a 28 day treatment cycle.
- After day 28, the next cycle will begin, in which you will again take this medicine once daily for five days followed by 23 days without TEMIZOLE.
- Before each new treatment cycle begins, your blood will be tested to determine if the TEMIZOLE dose needs to be adjusted.
TEMIZOLE alone (patients treated for recurrent brain tumour)
- Take the dose your doctor has prescribed once a day for five days.
- Depending on your response to TEMIZOLE, a new treatment cycle will begin each 28 days. You will then take this medicine again once daily for five days.
- Before each new treatment cycle, your blood will be tested to determine if the TEMIZOLE dose needs to be changed.
How to take TEMIZOLE
- Each time you start a new treatment cycle, be sure you understand exactly how many capsules of each strength you need to take on each day of dosing.
All patients
- TEMIZOLE comes in different strengths (shown on the label in ‘mg’). Each strength is a different colour. Depending on your dose of TEMIZOLE, you may have to take several capsules on each dosing day of the treatment cycle.
- Be sure you understand exactly how many capsules you need to take of each strength. Ask your doctor or pharmacist to write down the number of each strength (include colour) that you need to take on each dosing day.
- Be sure you know exactly which days are your dosing days.
- Be sure you review the dose with your doctor each time you start a new treatment cycle.
- Sometimes the dose or the mix of capsules you need to take will be different from the last cycle.
- Once you take this medicine home, if you are confused or unsure about how to take your dose, call your doctor or pharmacist before beginning the treatment cycle. Errors in how you take this medicine may have serious health consequences.
When to take TEMIZOLE
- Take your medicine on an empty stomach at least one hour before a meal.
- Swallow the capsules whole with a glass of water. Do not open or chew the capsules.
- If vomiting occurs after you take your medicine, do not take another dose that day.
How long to take TEMIZOLE
- Your doctor will tell you when your treatment should be stopped.
If you forget to use TEMIZOLE
TEMIZOLE should be used regularly at the same time each day. If you miss your dose at the usual time, take the missed dose as soon as possible during the same day. If a full day has gone by, check with your doctor.
Do not take a double dose to make up for the dose you missed.
If you use too much TEMIZOLE
If you think that you have used too much TEMIZOLE, you may need urgent medical attention.
You should immediately:
- phone the Poisons Information Centre
(Australia telephone 13 11 26) for advice, or - contact your doctor, or
- go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using TEMIZOLE?
Things you should do
Tell any other doctors, dentists and pharmacists who treat you that you are taking this medicine.
Tell your doctor if you feel sick or vomit while being treated with TEMIZOLE.
Your doctor may give you another medicine to help with this.
Tell your doctor if you become unusually pale or tired, get blood clotting problems or frequent infections while being treated with TEMIZOLE.
This could be caused by a low level of red blood cells, platelets or white blood cells in your blood. This is more common in patients over 70 years of age. Your doctor may need to change your dose of TEMIZOLE.
Tell your doctor immediately if you or your partner becomes pregnant while taking TEMIZOLE.
Be sure to keep all your doctor's appointments so your progress can be checked.
Your doctor may need to do some blood and other tests from time to time to check on your progress and detect any unwanted side effects.
Keep follow-up appointments with your doctor.
It is important to have your follow-up doses of TEMIZOLE at the appropriate times to get the best effects from your treatment.
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are taking TEMIZOLE.
Things you should not do
- Do not stop taking your medicine or change the dosage without checking with your doctor.
- Do not open the capsules. If a capsule is damaged, avoid contact with your skin, eyes and nose. Avoid inhaling the powder. If you touch the powder or get some in your eyes or nose, wash the area with water.
- Do not give your medicine to anyone else, even if they have the same condition as you.
- Do not take TEMIZOLE to treat any other complaints unless your doctor tells you to.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how TEMIZOLE affects you.
TEMIZOLE may cause drowsiness in some people. If this occurs, do not drive, operate machinery or do anything else that could be dangerous. Children should be careful when riding bicycles or climbing trees.
Looking after your medicine
- Keep your capsules in the bottle until it is time to take them.
- Keep your capsules in a cool dry place where the temperature stays below 25°C.
Follow the instructions in the carton on how to take care of your medicine properly.
Store it in a cool dry place away from moisture, heat or sunlight; for example, do not store it:
- in the bathroom or near a sink, or
- in the car or on window sills.
Keep it where young children cannot reach it.
When to discard your medicine
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering.
Getting rid of any unwanted medicine
If you no longer need to use this medicine or it is out of date, take it to any pharmacy for safe disposal.
Do not use this medicine after the expiry date.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of them are minor and temporary. However, some side effects may need medical attention.
Like other medicines used in the treatment of cancer, TEMIZOLE may have unwanted side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
See the information below and, if you need to, ask your doctor or pharmacist if you have any further questions about side effects.
Less serious side effects
| Less serious side effects | What to do |
| Speak to your doctor if you have any of these less serious side effects and they worry you. |
Serious side effects
| Serious side effects | What to do |
This could be caused by a low level of platelets or red blood cells in the blood.
| Call your doctor straight away, or go straight to the Emergency Department at your nearest hospital if you notice any of these serious side effects. |
Tell your doctor or pharmacist if you notice anything else that may be making you feel unwell.
Other side effects not listed here may occur in some people.
Reporting side effects
After you have received medical advice for any side effects you experience, you can report side effects to the Therapeutic Goods Administration online at www.tga.gov.au/reporting-problems. By reporting side effects, you can help provide more information on the safety of this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What TEMIZOLE contains
| Active ingredient (main ingredient) | Temozolomide |
| Other ingredients (inactive ingredients) |
The capsule shells contain:
|
| Potential allergens | TEMIZOLE contains sulfites and sugars as lactose. |
Do not take this medicine if you are allergic to any of these ingredients.
What TEMIZOLE looks like
TEMIZOLE comes in 5 strengths:
- TEMIZOLE 5 mg - Green/White hard gelatin capsules, size 3, imprinted 'TMZ' on the cap and '5' on the body containing white to light pink powder (AUST R 172973).
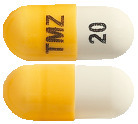
- TEMIZOLE 20 mg – Yellow/White hard gelatin capsules, size 5, imprinted 'TMZ' on the cap and '20' on the body containing white to light pink powder (AUST R 172968).
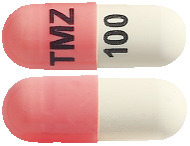
- TEMIZOLE 100 mg – Pink/White hard gelatin capsules, size 3, imprinted 'TMZ' on the cap and '100' on the body containing white to light pink powder (AUST R 172964).
- TEMIZOLE 140 mg – Transparent blue/White hard gelatin capsules, size 1, imprinted 'TMZ' on the cap and '140' on the body containing white to light pink powder (AUST R 172972).
- TEMIZOLE 250 mg – White/White hard gelatin capsules, size 0, imprinted 'TMZ' on the cap and '250' on the body containing white to light pink powder (AUST R 172975).
Each bottle contains 5 capsules.
Who distributes TEMIZOLE
Alphapharm Pty Ltd trading as Viatris
Level 1, 30 The Bond
30-34 Hickson Road
Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
This leaflet was prepared in July 2022.
TEMIZOLE® is a Viatris company trade mark
TEMIZOLE_cmi\Jul22/00
Published by MIMS September 2022

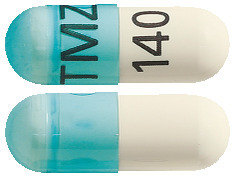







 Temozolomide is a white to pale brown/pink, crystalline powder which is odourless or almost odourless and hygroscopic. It is slightly soluble in water (3.1 mg/mL), methanol (4.4 mg/mL) and ethanol (0.6 mg/mL).
Temozolomide is a white to pale brown/pink, crystalline powder which is odourless or almost odourless and hygroscopic. It is slightly soluble in water (3.1 mg/mL), methanol (4.4 mg/mL) and ethanol (0.6 mg/mL).