WHAT IS IN THIS LEAFLET
This leaflet answers some common questions about Fycompa.
It does not contain all the available information. It does not take the place of talking to the doctor or pharmacist.
All medicines have risks and benefits. The doctor has weighed the risks of you or your child taking this medicine against the benefits they expect it will have for you or your child.
If you or your child have any concerns about taking this medicine, ask the doctor or pharmacist.
Keep this leaflet with the medicine. You may need to read it again.
WHAT FYCOMPA IS USED FOR
Fycompa is used to treat certain forms of epilepsy. In adults, adolescents and children aged 4 years and older.
- It is used to treat fits that affect one part of the brain (called a “partial seizure”). These partial seizures may or may not then be followed by a fit affecting all of the brain (called a “secondary generalisation”).
In adults, adolescents and children aged 7 years and older:
- It is also used to treat certain fits that affect all of the brain from the start and cause convulsions (called “primary generalised tonic-clonc seizures).
It contains the active ingredient perampanel. Perampanel belongs to a group of medicines called anti epileptics.
It works by reducing the number of fits that you or your children have.
Ask the doctor if you or your child have any questions about why this medicine has been prescribed for you or your child. The doctor may have prescribed it for another reason.
This medicine is available only with a doctor’s prescription.
Fycompa has some potential for abuse and misuse and should be used with caution in patients with a history of drug abuse.
There is not enough information to know whether Fycompa would be addictive if abused.
Fycompa should be used with caution in patients aged 65 years or older due to the high rates of dizziness and falls in those patients.
Fycompa should be used with caution in patients with a history of severe mental conditions or aggression.
There is not enough information to recommend the use of this medicine for children under the age of 4 years of age for partial seizures and under 7 years of age in primary generalised tonic clonic seizures.
BEFORE YOU OR YOUR CHILD TAKE FYCOMPA
When you or your child must not take it
Do not take this medicine if you or your child have an allergy to:
- Perampanel, the active ingredient, or to any of the other ingredients listed at the end of this leaflet under Product Description
Fycompa film coated tablets contain lactose. If you have been told by the doctor that you or your child have intolerance to some sugars, tell the doctor before taking it.
Fycompa oral suspension contains sorbitol and benzoates. If you have been told by the doctor that you or your child have intolerance to these, tell the doctor before taking it.
Some of the symptoms of an allergic reaction may include:
- shortness of breath
- wheezing or difficulty breathing
- swelling of the face, lips, tongue or other parts of the body
- rash, itching or hives on the skin
Do not give this medicine to a child under the age of 4 years. The safety and effectiveness are not yet known in children under 4 years of age for partial seizures and under 7 years of age in primary generalised tonic clonic seizures.
Do not take this medicine after the expiry date printed on the pack or if the packaging is torn or shows signs of tampering. If it has expired or is damaged, return it to a pharmacy for disposal.
If you or your child are not sure whether to start taking this medicine, talk to the doctor.
Before you or your child start to take it
Tell the doctor if you or your child has allergies to any other medicines, foods, preservatives or dyes.
Tell the doctor if you or your child has or have had any of the following medical conditions:
- liver problems
- moderate or severe kidney problems
- history of alcoholism, drug dependence or other psychiatric illness
Tell the doctor if you are pregnant or plan to become pregnant or are breastfeeding. Fycompa is not recommended in pregnancy.
You must use a reliable method of contraception to avoid becoming pregnant while being treated with Fycompa.
You should continue doing this for one month after stopping treatment.
Tell the doctor if you are taking hormonal contraceptives. Fycompa may make certain hormonal contraceptives such as levonorgestrel less effective.
You should use other forms of safe and effective contraception (such as a condom or coil) when taking Fycompa. You should also do this for one month after stopping treatment. Discuss with the doctor what may be appropriate contraception for you.
It is not known whether the ingredients of Fycompa can pass into breast milk.
The doctor will weigh up the benefit and risks to you or your baby of taking Fycompa while you are breastfeeding.
If you or your child have not told the doctor about any of the above, tell him/her before you or your child start taking Fycompa.
Taking other medicines
Tell the doctor or pharmacist if you or your child are taking any other medicines, including any that you or your child get without a prescription from the pharmacy, supermarket or health food shop.
Some medicines and Fycompa may interfere with each other. These include:
- Carbamazepine, phenytoin, oxcarbazepine
- Rifampicin,
- Hypericum (St. John’s wort),
- Felbamate
- Ketoconazole
- Oral contraceptives
These medicines may be affected by Fycompa or may affect how well it works. You or your child may need different amounts of medicines, or may need to take different medicines.
The doctor and pharmacist have more information on medicines to be careful with or avoid while taking this medicine.
HOW TO TAKE FYCOMPA
Follow all directions given to you or your child by the doctor or pharmacist carefully. They may differ from the information contained in this leaflet.
If you or your child do not understand the instructions, ask the doctor or pharmacist for help.
How much to take
In adults and adolescents, the usual starting dose for this medicine is 2 mg (4 mL of oral suspension) once a day before you go to bed.
The doctor may increase the dose in 2 mg steps (4 mL of oral suspension) to a maintenance dose between 4 and 12 mg depending on you or your child’s response.
The doctor may have prescribed a different dose.
In children less than 12 years old, the starting dose for this medicine will be based on you or your childs age and weight. The usual starting dose is 1 or 2 mg (2 mL or 4 mL of oral suspension) once a day before going to bed.
The doctor may increase the dose in 1 mg or 2 mg steps (2 mL or 4 mL of oral suspension) to a maintenance dose between 2 and 12 mg depending on the response.
In adults and adoelscents with mild or moderate liver problems, dosing can be started a the normal starting dose and the dose increases should be at least 2 weeks apart. Dosing should not exceed the maintenance dose.
In children less than 12 years old with mild or moderate liver problems, there are no dosing recommendations.
The doctor may have prescribed a different dose.
Ask the doctor or pharmacist if you or your child are unsure of the correct dose. They will tell you or your child exactly how much to take.
Follow the instructions they give you or your child. If you or your child take the wrong dose, Fycompa may not work as well and the problem may not improve.
How to take it
You or your child can take Fycompa with or without food and should always take it the same way. For example, if you or your child decide to take Fycompa with food, always take it that way.
Film coated tablets
Swallow the tablets whole with a full glass of water. You or your child can take Fycompa tablets with or without food.
Do not chew, crush or split the tablet. The tablets cannot be split accurately as there is no break line. To ensure you or your child get the entire dose, the tablets should be swallowed whole without chewing or crushing.
Oral suspension
Instructions on how to use the oral syringe and adaptor are provided below:

- Shake the oral suspension for at least 5 seconds before every administration.

- Push down and turn cap to open bottle.

- Insert adaptor into the neck of the bottle until a tight seal is made.

- Push plunger of oral syringe completely down.
- Insert the oral syringe into the opening of the adaptor as far as possible.

- Turn upside down and withdraw the prescribed amount of Fycompa from the bottle.

- Turn upright and remove the oral syringe.
- Leave the adaptor in place and replace cap on bottle. Wash the oral syringe with clean water and dry thoroughly.
When to take Fycompa
Take the medicine at about the same time each day before going to bed. Taking it at the same time each day will have the best effect. It will also help you or your child remember when to take it.
How long to take Fycompa
Continue taking the medicine for as long as the doctor tells you or your child.
Do not stop unless the doctor advises you or your child to.
The doctor may reduce the dose slowly to avoid fits (seizures) coming back or getting worse.
If you or your child have any further questions on the use of this medicine, ask the doctor or pharmacist.
If you or your child forget to take it
Wait until the next dose, and then continue to take it as you or your child would normally.
Do not take a double dose to make up for the missed dose. This may increase the chance of you or your child getting an unwanted side effect.
If you or your child have missed less than 7 days of treatment with Fycompa, continue taking the daily dose as originally instructed by the doctor.
If you or your child have missed more than 7 days of treatment with Fycompa, talk to the doctor immediately.
If you or your child are not sure what to do, ask the doctor or pharmacist.
If you or your child have trouble remembering to take the medicine, ask the pharmacist for some hints.
If you or your child take too much (overdose)
Immediately telephone the doctor or the Poisons Information Centre (telephone Australia 13 11 26 for advice, or go to Accident and Emergency at the nearest hospital, if you or your child think that you or your child or anyone else may have taken too much Fycompa. Do this even if there are no signs of discomfort or poisoning. You or your child may need urgent medical attention.
WHILE YOU OR YOUR CHILD ARE TAKING FYCOMPA
Things you or your child must do
If you or your child are about to be started on any new medicine, remind the doctor and pharmacist that you or your child are taking Fycompa.
Tell any other doctors, dentists and pharmacists who treat you or your child that you or your child are taking Fycompa.
If you or your child are going to have surgery, tell the surgeon or anaesthetist that you or your child are taking Fycompa. It may affect other medicines used during surgery.
If you or your child become pregnant while taking Fycompa, tell the doctor immediately. Do not stop treatment without first discussing it with the doctor.
If you or your child are about to have any blood tests, tell the doctor that you or your child are taking Fycompa. It may interfere with the results of some tests.
Keep all of the doctor’s appointments so that you or your child’s progress can be checked.
Things you or your child must not do
Do not take Fycompa to treat any other complaints unless the doctor tells you or your child to.
Do not give you or your child’s medicine to anyone else, even if they have the same condition as you or your child.
Do not stop taking the medicine or lower the dosage without checking with the doctor.
Things to be careful of
Serious or life-threatening psychiatric and behavioural adverse reactions including aggression, hostility, irritability, anger, suicidal ideation and homicidal ideation and threats have been reported in patients taking Fycompa.
Patients and caregivers should contact a healthcare provider immediately if any of these reactions or changes in mood, behaviour, or personality that are not typical for the patient are seen while they are taking Fycompa or after stopping Fycompa.
If changes in behaviour or personality are seen notify the doctor immediately. They may reduce the dose of Fycompa or stop treatment with Fycompa.
Patients starting Fycompa should be carefully observed especially when starting treatment and if the dose is increased.
Do not drive or operating machinery until you know how Fycompa affects you or your child.
You must talk to the doctor about the effect of you or your child’s epilepsy on driving and using machines.
Fycompa may make you or your child feel dizzy or sleepy, particularly at the beginning of treatment. If this happens to you or your child, do not drive or use any tools or machines.
Fycompa may make you or your child more likely to fall over, particularly if you are an older person; this might be due to you or your child’s illness.
Avoid alcohol while taking Fycompa as it may make these effects worse.
SIDE EFFECTS
Tell the doctor or pharmacist as soon as possible if you or your child do not feel well while you or your child are taking Fycompa
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You or your child may need medical attention if you or your child get some of the side effects.
Do not be alarmed by the following lists of side effects. You or your child may not experience any of them.
A small number of people being treated with antiepileptics have had thoughts of harming or killing themselves. If at any time you or your child have these thoughts, contact the doctor or go to accident & emergency straight away.
If you or your child get a skin rash, or fever, and/or enlarged lymph nodes, these could be signs of an allergic reaction. See the doctor immediately as very occasionally this may become serious.
Very common (may affect more than 1 user in 10) side effects are:
- feeling dizzy
- feeling sleepy (drowsiness or somnolence).
Common (may affect more than 1 user in 100) side effects are:
- increased or decreased appetite, weight gain
- feeling aggressive, angry, irritable, anxious or confused
- difficulty with walking or other balance problems (ataxia, gait disturbance, balance disorder)
- slow speech (dysarthria)
- blurred vision or double vision (diplopia)
- spinning sensation (vertigo)
- feeling sick (nausea)
- back pain
- feeling very tired (fatigue)
- falling down.
Not known (the frequency of this side effect cannot be estimated from the available data) are:
- thoughts about harming yourself or your child having thoughts about harming themselves;thoughts about ending your own life or your child having thoughts about ending their own life (suicidal thoughts), tried to end your own life or your child tried to end their own life (attempted suicide)
- skin rash
- fever
- enlarged lymph nodes
Children are more likely than adults and adolescents to feel sleepy, or feel aggressive, angry, irritable, anxious or confused.
If you or your child get any side effects, talk to the doctor or pharmacist.
This includes any possible side effects not listed in this leaflet.
Ask the doctor or pharmacist to answer any questions you or your child may have.
Tell the doctor or pharmacist if you or your child notice anything else that is making you or your child feel unwell. Other side effects not listed above may also occur in some people.
AFTER TAKING FYCOMPA
Storage
Keep the medicine in the original container.
If you or your child take it out of its original container it may not keep well.
Keep the medicine in a cool dry place where the temperature stays below 30°C. Do not freeze the oral suspension. If there is any suspension left in the bottle more than 90 days after it was first opened, it should not be used.
Do not store Fycompa or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep it where children cannot reach it. A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If the doctor tells you or your child to stop taking this medicine or the expiry date has passed, ask the pharmacist what to do with any medicine that is left over.
PRODUCT DESCRIPTION
What it looks like
Film coated tablets
Fycompa 2 mg film coated tablet is an orange, round, biconvex film-coated tablet, engraved with E275 on one side and 2 on other side. Available in packs of 7.
Fycompa 4 mg film coated tablet is a red, round, biconvex film-coated tablet, engraved with E277 on one side and 4 on other side. Available in packs of 28.
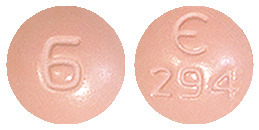
Fycompa 6 mg film coated tablet is a pink, round, biconvex film-coated tablet, engraved with E294 on one side and 6 on other side. Available in packs of 28.
Fycompa 8 mg film coated tablet is a purple, round, biconvex film-coated tablet, engraved with E295 on one side and 8 on other side. Available in packs of 28.
Fycompa 10 mg film coated tablet is a green, round, biconvex film-coated tablet, engraved with E296 on one side and 10 on other side. Available in packs of 28.
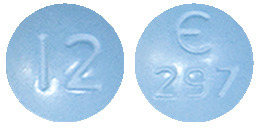
Fycompa 12 mg film coated tablet is a blue, round, biconvex film-coated tablet, engraved with E297 on one side and 12 on other side. Available in packs of 28.
Oral suspension
FYCOMPA oral suspension appears as a white to off white suspension. Available in packs of 1 bottle containing 340 mL oral suspension.
Ingredients
Film coated tablets
Active ingredient:
- Perampanel (as hemisesquihydrate)
Excipient Ingredients:
- lactose,
- hypromellose,
- povidone,
- purified talc,
- magnesium stearate,
- microcrystalline cellulose (6 mg, 8 mg, 10 mg and 12 mg only)
- macrogol 8000,
- titanium dioxide,
- iron oxide yellow (2 mg, 10 mg),
- iron oxide red (2mg, 4 mg, 6 mg, and 8 mg only),
- iron oxide black (8 mg only) and
- Indigo carmine aluminium lake (10 mg & 12 mg only).
This medicine does not contain gluten, tartrazine or any other azo dyes.
Oral suspension
Active ingredient:
- Perampanel (as hemisesquihydrate)
Excipient Ingredients:
- sorbitol solution (70%) (crystallising),
- Avicel RC – 591 (PING: 4093)
- poloxamer,
- Antifoam AF Emulsion Q7 - 2587 (PING: 1515)
- citric acid,
- sodium benzoate,
- purified water
This medicine does not contain gluten, tartrazine or any other azo dyes.
SPONSOR
Eisai Australia Pty. Ltd.
Level 2, 437 St Kilda Road
Melbourne, VIC, 3004
[email protected]
This leaflet was prepared February 2021.
Australian Register Number(s)
Fycompa 2 mg film coated tablet: AUST R 207690
Fycompa 4 mg film coated tablet: AUST R 207689
Fycompa 6 mg film coated tablet: AUST R 207688
Fycompa 8 mg film coated tablet: AUST R 207687
Fycompa 10 mg film coated tablet: AUST R 207692
Fycompa 12 mg film coated tablet: AUST R 207691
Fycompa 4 mg/2mL oral suspension: AUST R 332505
Published by MIMS April 2021






 The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other indications, but the absolute risk differences were similar for epilepsy and psychiatric conditions.
The relative risk for suicidal thoughts or behaviour was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other indications, but the absolute risk differences were similar for epilepsy and psychiatric conditions. Based on the results from the population pharmacokinetic analysis of patients with partial onset seizures and patients with primary generalised tonic-clonic seizures, the total clearance of Fycompa was increased when co-administered with carbamazepine (3-fold), and phenytoin or oxcarbazepine (2-fold), which are known inducers of enzymes of metabolism (see Section 5.2 Pharmacokinetic Properties). This effect should be taken into account and managed when adding or withdrawing these AEDs from a patient's treatment regimen. Clonazepam, levetiracetam, phenobarbital, topiramate, zonisamide, clobazam, lamotrigine and valproic acid did not affect to a clinically relevant manner the clearance of Fycompa.
Based on the results from the population pharmacokinetic analysis of patients with partial onset seizures and patients with primary generalised tonic-clonic seizures, the total clearance of Fycompa was increased when co-administered with carbamazepine (3-fold), and phenytoin or oxcarbazepine (2-fold), which are known inducers of enzymes of metabolism (see Section 5.2 Pharmacokinetic Properties). This effect should be taken into account and managed when adding or withdrawing these AEDs from a patient's treatment regimen. Clonazepam, levetiracetam, phenobarbital, topiramate, zonisamide, clobazam, lamotrigine and valproic acid did not affect to a clinically relevant manner the clearance of Fycompa.
 TEAEs leading to dose reduction were observed in 40.6% of children. Common TEAEs leading to dose reduction in children included somnolence (13.3%), dizziness (5.6%), irritability (4.4%), and aggression (4.4%). TEAEs leading to discontinuation were observed in 9.4% of children. Reasons for discontinuation occurring in more than one subject were irritability (1.7%), aggression (1.7%), seizure (1.1%), and balance disorder (1.1%).
TEAEs leading to dose reduction were observed in 40.6% of children. Common TEAEs leading to dose reduction in children included somnolence (13.3%), dizziness (5.6%), irritability (4.4%), and aggression (4.4%). TEAEs leading to discontinuation were observed in 9.4% of children. Reasons for discontinuation occurring in more than one subject were irritability (1.7%), aggression (1.7%), seizure (1.1%), and balance disorder (1.1%). Therefore the number needed to treat (NNT) with any dose of Fycompa for 4 mg to 12 mg to achieve a 50% reduction in seizure frequency was 6.25 to 10.9.
Therefore the number needed to treat (NNT) with any dose of Fycompa for 4 mg to 12 mg to achieve a 50% reduction in seizure frequency was 6.25 to 10.9. Following 23 weeks of perampanel treatment, 42.6% of patients with partial-onset seizures, 43.7% in the subset of partial-onset seizure patients with secondarily generalized seizures, 34.8% of patients with primary generalized tonic-clonic seizures, and 35.3% in the subset of primary generalized tonic-clonic seizures of idiopathic generalized epilepsy (IGE) patients were very much improved or much improved compared to baseline, as assessed by Clinical Global Impression of Change (CGIC). The treatment effects on the CGIC observed above were sustained following 52 weeks of perampanel treatment.
Following 23 weeks of perampanel treatment, 42.6% of patients with partial-onset seizures, 43.7% in the subset of partial-onset seizure patients with secondarily generalized seizures, 34.8% of patients with primary generalized tonic-clonic seizures, and 35.3% in the subset of primary generalized tonic-clonic seizures of idiopathic generalized epilepsy (IGE) patients were very much improved or much improved compared to baseline, as assessed by Clinical Global Impression of Change (CGIC). The treatment effects on the CGIC observed above were sustained following 52 weeks of perampanel treatment.
