Biologics, biosimilars and PBS sustainability
Biological medicines are used to treat many diseases, and account for a high proportion of expenditure on the PBS. Biosimilars have the same safety, effectiveness and quality as their reference biologics. Find out more about how they provide a driver for market competition and contribute to a sustainable PBS.

Biologics have a significant and positive impact on the treatment of many severe acute and chronic diseases. In the 2019–20 financial year, biologics accounted for 7 of the top 10 Pharmaceutical Benefits Scheme (PBS) subsidised medicines by expenditure.1 After the patents on the original (reference) biologics expire, competing manufacturers are able to develop biosimilars, which are highly similar versions of a specific reference biologic (sometimes called the ‘originator’ biologic). Once this happens, market competition usually drives prices down.
Key points
- Biologics play an important role in therapy for many people.
- Biosimilars are highly similar versions of biologics.
- Therapeutic outcomes and adverse effects for biologics and biosimilars are the same.
- Timely availability and utilisation of biosimilars contributes to a sustainable PBS with equivalent therapeutic outcomes.
Biosimilars, competition and the PBS
Biologics are used in the treatment of many acute and chronic conditions, in the areas of rheumatology, gastroenterology, dermatology, endocrinology, oncology and haematology, among others. These medicines are relatively expensive to develop and manufacture, compared to conventional medicines, and account for a high proportion of expenditure on the PBS.1
A challenge for the PBS is to achieve a balance between allowing access to new and innovative medicines and containing costs.2 After the patents on reference biologics expire, competing manufacturers are able to market biosimilars, which are highly similar versions of a reference (sometimes called ‘originator’) biologic. This provides a driver for market competition and potential savings for the PBS.3
How does the PBS decide to list new medicines?
For a new medicine to be subsidised on the PBS, it must first be considered by the Pharmaceutical Benefits Advisory Committee (PBAC). When a sponsor (usually a pharmaceutical company) makes a submission for a medicine to be listed on the PBS, the PBAC considers the relative clinical and economic merits of the new medicine against a nominated ‘comparator’, or multiple comparators, when reaching a decision. These comparators may be other drug or non-drug therapies.8 To list a medicine, the PBAC must decide that the medicine is safe and effective and also cost-effective.9
After assessing the submission, PBAC makes either a positive or negative recommendation to the Australian Government Minister for Health, who has the authority to list the medicine on the PBS.
Once the PBAC recommends listing a medicine on the PBS, pricing negotiations take place between the sponsor and the Department of Health and the details for listing the medicine on the PBS are finalised.10
How is cost-effectiveness established?
Several methods inform funding decisions in health care. These include cost‑effectiveness, cost-utility, cost-minimisation and cost–benefit analysis.2 The PBAC assesses the benefits of funding decisions relative to the cost when making recommendations for medicines to government.
The economic basis of a PBAC recommendation usually follows one of two approaches:
- A cost-effectiveness analysis allows a proposed new medicine to be listed with a price advantage over its comparator, provided the extent of this price advantage can be justified by improved health outcomes, efficacy or safety.8
- A cost-minimisation approach applies where there are insufficient gains in health outcomes to justify a higher price for the proposed medicine over currently listed alternatives.8
For further information on these different methods see the Australian Prescriber article Economic evaluation of medicines.
Why are reference biologics and their biosimilars reimbursed the same on the PBS?
A government pricing policy, called reference pricing applies where new medicines considered to be of similar safety and efficacy are linked for pricing purposes and recommended by the PBAC as cost-minimised.11,12 This applies to biosimilars of reference biologics. That is, the lowest-priced medicine listed on the PBS sets a benchmark price for the other brands of that medicine.11
How does prescribing biosimilars contribute to a sustainable PBS?
After listing, the Government has mechanisms, including statutory price reductions and price disclosure, to ensure , that the PBS remains affordable for the community while providing access to necessary medicines and maintaining a viable pharmaceutical industry.13,14
What is the effect of statutory price reductions?
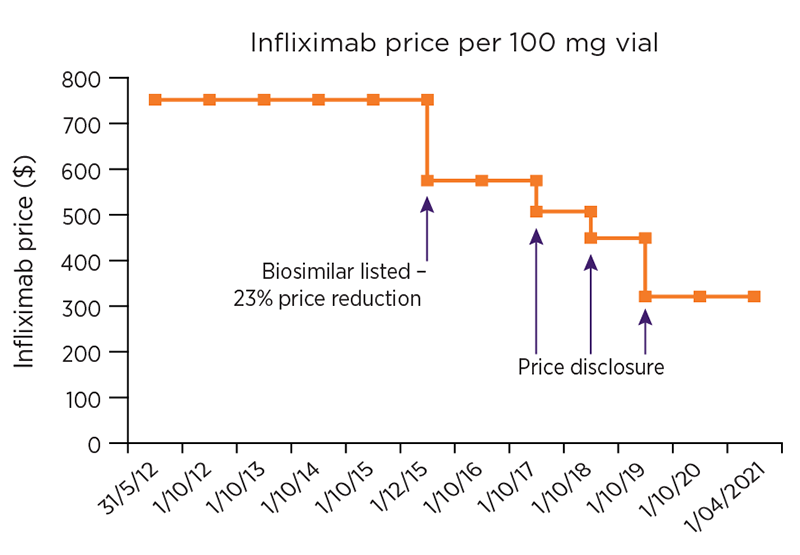
When biologics are first approved on the PBS, they are listed on the F1 formulary (F1), which consists of single-brand medicines, usually with patent protection.15 The prices of medicines on F1 are generally stable9 and are reduced on the 5-, 10- and 15-year anniversaries of their listing, for as long as they remain on F1. These reductions are required by law and are known as statutory price reductions. When a biosimilar enters the market, the medicine is generally moved to the F2 formulary (F2), which consists of multi-brand medicines, such as generics and biosimilar medicines.15 At this time, the original reference biologic is subject to a one-off price reduction of up to 25%.16
How does market competition influence price?
Once a medicine is listed on F2, market competition usually drives prices down. A pricing mechanism called ‘price disclosure’, allows the government to benefit from this competition and reduce the price of PBS medicines in some instances, to reflect the prices for which the medicine is being sold in the market. Price disclosure cycles occur every 6 months and multiple successive reductions can apply to a medicine for as long as it is listed on F2.
To enable this, manufacturers are required to provide the Department of Health with their ex-manufacturer price at time of supply to wholesalers and pharmacies (excluding public hospital sales). If the average ex-manufacturer price, is more than 10% (or 30% in some cases) below the ex-manufacturer price listed on the PBS, then the listed price will be reduced. This avoids a situation where the Government would otherwise end up subsidising medicines for more than the price at which they are actually being sold by wholesalers to pharmacies.
Figure 1a: Changes to ex-manufacturer price of etanercept PBS listing
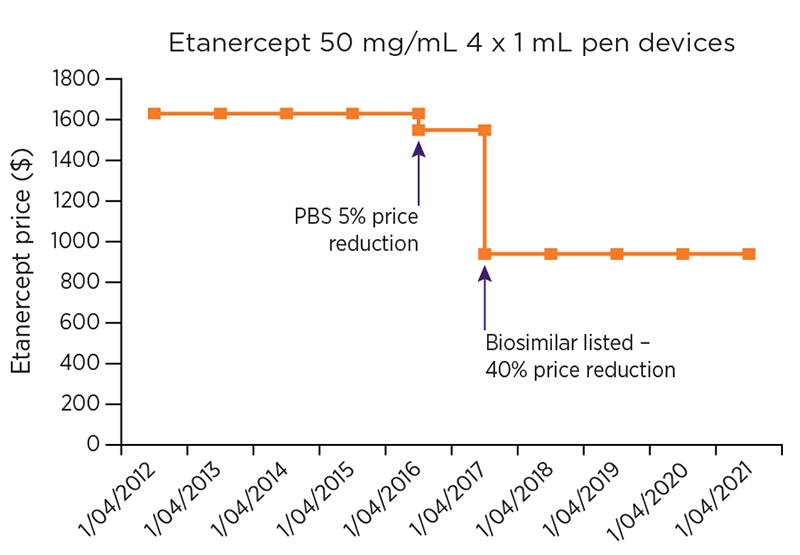
Figure 1b: Changes to ex-manufacturer price of infliximab PBS listing
Data included in these graphs was obtained from publicly available information on the PBS website
Read an accessible version of the data in these graphs
The price disclosure calculation accounts for both the level of discounting and the volume of products sold. By increasing the prescribing of biosimilars, there is increased competition of comparable products, and consequently prices may decrease, which are then reflected in further price disclosure cycles. As a result, the Australian government is able to reinvest any savings into the PBS, providing increased choice and availability for consumers and health professionals.
What are the benefits of biosimilar medicines?
The availability of biosimilars in Australia has decreased the cost of individual biologics (see Figure 1) and consequently could also potentially:
- improve cost-effectiveness of biologics in various conditions
- improve economic efficiencies by creating a more competitive market with a broader range of cost-effective treatment options
- contribute to ongoing PBS sustainability and reinvestment in new treatments
- expand access to medicines via broader eligibility criteria or broadening of approved indications
- improve the security of the supply chain, ensuring fewer consumers are affected by medicine shortages.3,13,18-20
Biosimilars lower the ongoing PBS expenditure on biologics, and a sustainable PBS keeps the system affordable both for individuals and for Australian society as a whole.
Prescribing of biosimilars may also save time for prescribers, due to the availability of streamlined authorities which the reference biologic may not have.
“Overall, the introduction of biosimilar agents has the potential to widen patient access to effective biologic therapy, to better accommodate restraints within healthcare budgets and to improve overall patient outcomes.”21
Prescribing considerations
The prescriber, determines, based on clinical and individual factors and in consultation with the patient, which medicine is the most appropriate for each individual patient. The choice of the medicine is also influenced by the cost of the medicine and its PBS availability, including criteria and restrictions.
If the prescriber decides to initiate a PBS-listed biologic, for an approved rheumatological, gastroenterological or dermatological condition, then they decide which class of medicine to use, and which medicine within that class. At this stage, the prescriber may consider the choice of brand of medicine to prescribe.
The choice of reference or biosimilar brand may be influenced by:
- delivery method
- device for delivery
- patient support program if appropriate
- authority process.
A biosimilar brand may be chosen at initiation for a biologic-naïve consumer, or a consumer may be switched to a biosimilar brand. As part of the Biosimilar Awareness Initiative, two specific uptake drivers are being implemented:
- encouraging prescribing of a biosimilar brand rather than the reference biologic brand for treatment-naïve consumers
- providing a simpler and faster authority approval process for biosimilar brands (eg, streamlined authority) while maintaining an existing higher level authority requirement for the reference biologic brand (eg, written authority).13
This may result in time savings for the prescriber and facilitate easier coordination of access to medicines for the consumer.
In line with active ingredient prescribing, a biologic should be prescribed by active ingredient first. A prescriber can still include a brand name if they consider it clinically appropriate. To avoid inadvertent switching, prescribers may add in the brand name, and tick the ‘Brand substitution not permitted’ box.
For further information on active ingredient prescribing:
References
- PBS Information Management Section, Pricing and PBS Policy Branch. PBS Expenditure and Prescriptions Report 1 July 2019 to 30 June 2020. Canberra: Australian Government Department of Health, 2020 (accessed 7 April 2021).
- Taylor C, Jan S. Economic evaluation of medicines. Aust Prescr 2017;40:76-8.
- Australian Government Department of Health. Why are biosimilar medicines important? Canberra: DoH, 2017 (aaccessed 15 March 2021).
- Australian Government Department of Health. What are biosimilar medicines? Canberra: DoH, 2017 (accessed 15 March 2021).
- Therapeutic Goods Administration. Biosimilar medicines regulation. Canberra: Australian Government Department of Health, 2018 (accessed 29 July 2020).
- Australian Government Department of Health. Is there a difference in health outcomes between the biosimilar medicine and the reference biological medicine? Canberra: DoH, 2017 (accessed 15 March 2021).
- Cohen HP, Blauvelt A, Rifkin RM, et al. Switching reference medicines to biosimilars: A systematic literature review of clinical outcomes. Drugs 2018;78:463-78.
- Pharmaceutical Benefits Scheme. The effects of statutory price reductions on the listing of new medicines. Canberra: Australian Government Department of Health, 2021 (accessed 17 February 2021).
- GSK Australia, ViiV Healthcare. The pharmaceutical benefits scheme in Australia: an explainer on system components. Sydney: GSK Australia, 2018.
- Pharmaceutical Benefits Advisory Committee. PBAC outcomes. Canberra: Australian Government Department of Health, 2021 (accessed 10 March 2021).
- Pharmaceutical Benefits Scheme. Fact sheet – Setting an approved ex-manufacturer price for new or extended listings. Canberra: Australian Government Department of Health, 2017 (accessed 17 February 2021).
- Australian Government. National Health Act 1953 Subsection 85C. Canberra: Parliament of Australia, 2020 (accessed 17 February 2021).
- Pharmaceutical Benefits Scheme. Biosimilar Uptake Drivers. Canberra: Australian Government, Department of Health, 2020 (accessed 15 March 2021).
- Pharmaceutical Benefits Scheme. About the PBS. Canberra: Australian Government Department of Health, 2021 (accessed 15 March 2021).
- Pharmaceutical Benefits Scheme. Formulary Allocations - 1 March 2021. Canberra: Australian Government Department of Health, 2021 (accessed 21 March 2021).
- Pharmaceutical Benefits Scheme. First New Brand Price Reductions. Canberra: Australian Government, Department of Health, 2021 (accessed 15 March 2021).
- Pharmaceutical Benefits Scheme. Manual of resource items and their associated unit costs. Canberra: Australian Government Department of Health, 2016 (accessed 15 March 2021).
- Council of Australian Therapeutic Advisory Groups. Biologics and biosimilars best practice: Guiding principles for the governance of biologics and their biosimilars in Australian hospitals. Sydney: CATAG, 2021 (accessed 15 March 2021).
- Australian Government Department of Health. Biosimilar Awareness Initiative. Canberra: DoH, 2020 (accessed 15 March 2021).
- Haifer C, Srinivasan A, An YK, et al. Switching Australian patients with moderate to severe inflammatory bowel disease from originator to biosimilar infliximab: a multicentre, parallel cohort study. Med J Aust 2021;214:128-33.
- Schulze-Koops H, Skapenko A. Biosimilars in rheumatology: A review of the evidence and their place in the treatment algorithm. Rheumatology (Oxford) 2017;56:iv30-iv48.
