Key points
- From 1 July 2018, adrenaline (epinephrine) 300 microgram and 150 microgram autoinjectors (brand name Emerade) became available on the Pharmaceutical Benefits Scheme (PBS).1
- An adrenaline autoinjector is used for the emergency treatment of anaphylaxis (acute severe allergic reaction).1
- Prescribers, pharmacists and consumers can access information about Emerade brand autoinjectors, including method of administration and labelling by visiting the Australasian Society of Clinical Immunology and Allergy – ASCIA website.
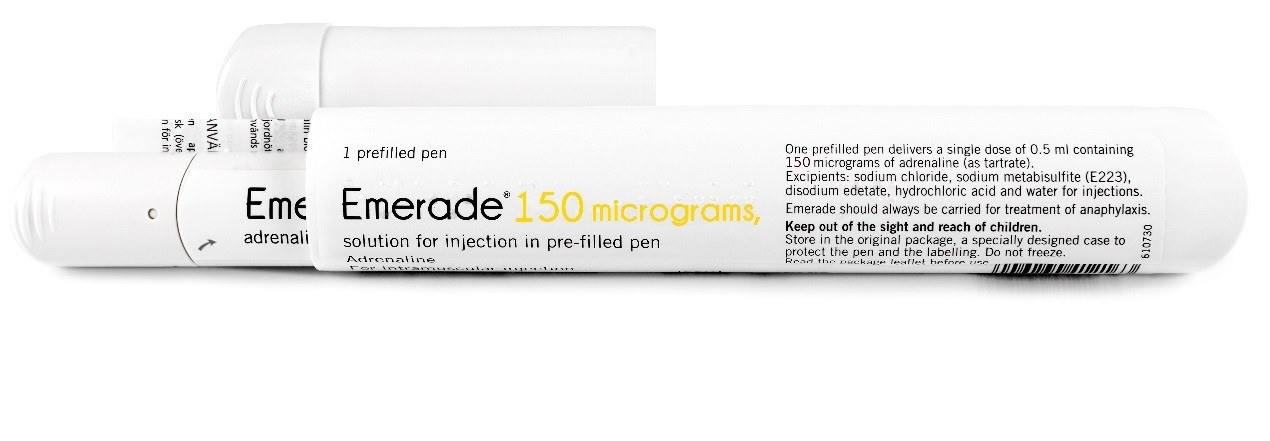
On 1 July 2018, both autoinjector formulations (Figures 1 a and b) became available on the PBS.1
Be mindful of product labelling differences
There are now three 150 microgram adrenaline autoinjectors listed on the PBS:3
- EpiPen Jr and ‘a’ flagged brand equivalent Adrenaline Jr Mylan
- Emerade 150 microgram autoinjector.
It is important to note that Emerade 150 microgram autoinjector labelling does not specify Jr on the label (Figure 1a). Always read the full product labelling to confirm the correct formulation of adrenaline (epinephrine) before administering.
Mode of administration differences
Emerade autoinjectors have a different mode of administration to EpiPen/Adrenaline Mylan autoinjectors. It is recommended that these different products are not prescribed to the same person unless appropriate training is provided.1,3
It is important for prescribers and pharmacists to ensure their patients are familiar with the mode of administration for the autoinjector they have been prescribed.
Dispensing a different brand of adrenaline autoinjector provides an opportunity for discussion and review of the patient’s Anaphylaxis Action Plan.
Ensure others who may also be responsible for delivering emergency anaphylaxis using an autoinjector are aware of the administration differences between EpiPen/Adrenaline Mylan products and Emerade products. This may include childcare providers and schools.
Prescribers, pharmacists and consumers can access information about How to give Emerade, including method of administration and labelling by visiting the ASCIA website.
Key points for consumers
- From 1 July 2018, Emerade 150 microgram adrenaline autoinjector became available on the PBS for emergency anaphylaxis treatment - this dose is often prescribed for children who weigh between 10 kg and 20 kg. Unlike the EpiPen and Adrenaline Mylan products, the Emerade labelling does not specify Jr on the 150 microgram autoinjector. If you are using Emerade products always check the label to make sure you are using the correct dose.
- From 1 July 2018, Emerade 300 microgram adrenaline autoinjector became available on the PBS for emergency anaphylaxis treatment - this dose is often prescribed for children over 20 kgs and adults.
- Discuss your personal anaphylaxis needs with your general or nurse practitioner.
- Read instructions for How to give Emerade on the ASCIA website.
- Obtaining a script for a different brand of adrenaline autoinjector provides an opportunity for discussion and review of your Anaphylaxis Action Plan.
References
- Pharmaceutical Benefits Scheme (PBS). Schedule of Pharmaceutical Benefits - Summary of Changes. Effective 1 July 2018. Canberra: PBS, 2018 (accessed 3 July 2018).
- Therapeutic Goods Administration. Database of section 19A approvals to import and supply medicines to address medicine shortages. Canberra: TGA (accessed 6 June 2018).
- Pharmaceutical Benefits Scheme. Adrenaline (epinephrine) Canberra: PBS (accessed 30 May 2018).


