Non-vitamin K antagonist oral anticoagulants
Apixaban, dabigatran and rivaroxaban (formerly known as NOACsa) are non-vitamin K antagonist oral anticoagulants currently listed on the PBS.
Apixaban and rivaroxaban are selective inhibitors of factor Xa that block thrombin production, conversion of fibrinogen to fibrin, and thrombus development.1 Dabigatran is a competitive and reversible direct thrombin inhibitor preventing thrombus formation.1
Non-vitamin K antagonist oral anticoagulants are TGA registered and PBS listed for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF) with one or more risk factors for stroke (Figure 1). Other indications include the prevention of venous thromboembolism, deep vein thrombosis and pulmonary embolism.2,3
Apixaban, dabigatran and rivaroxaban have been shown to be as effective as warfarin in reducing the risk of stroke in patients with NVAF.4
Drug Utilisation Sub Committee (DUSC) data on PBS utilisation of apixaban, dabigatran and rivaroxaban show that use of warfarin has declined since the listing of these medicines on the PBS for NVAF.2
The analysis also reported that patients starting anticoagulant therapy for the first time are now more likely to start one of these three medicines than warfarin.2
Despite the growth in the use of apixaban, dabigatran and rivaroxaban, there are some concerns about their safety due to the risk of major bleeding (Figure 1).2 As with other anticoagulants, clinical trials and post-market experience have shown major bleeding events, including those leading to death associated with apixaban, dabigatran and rivaroxaban.5
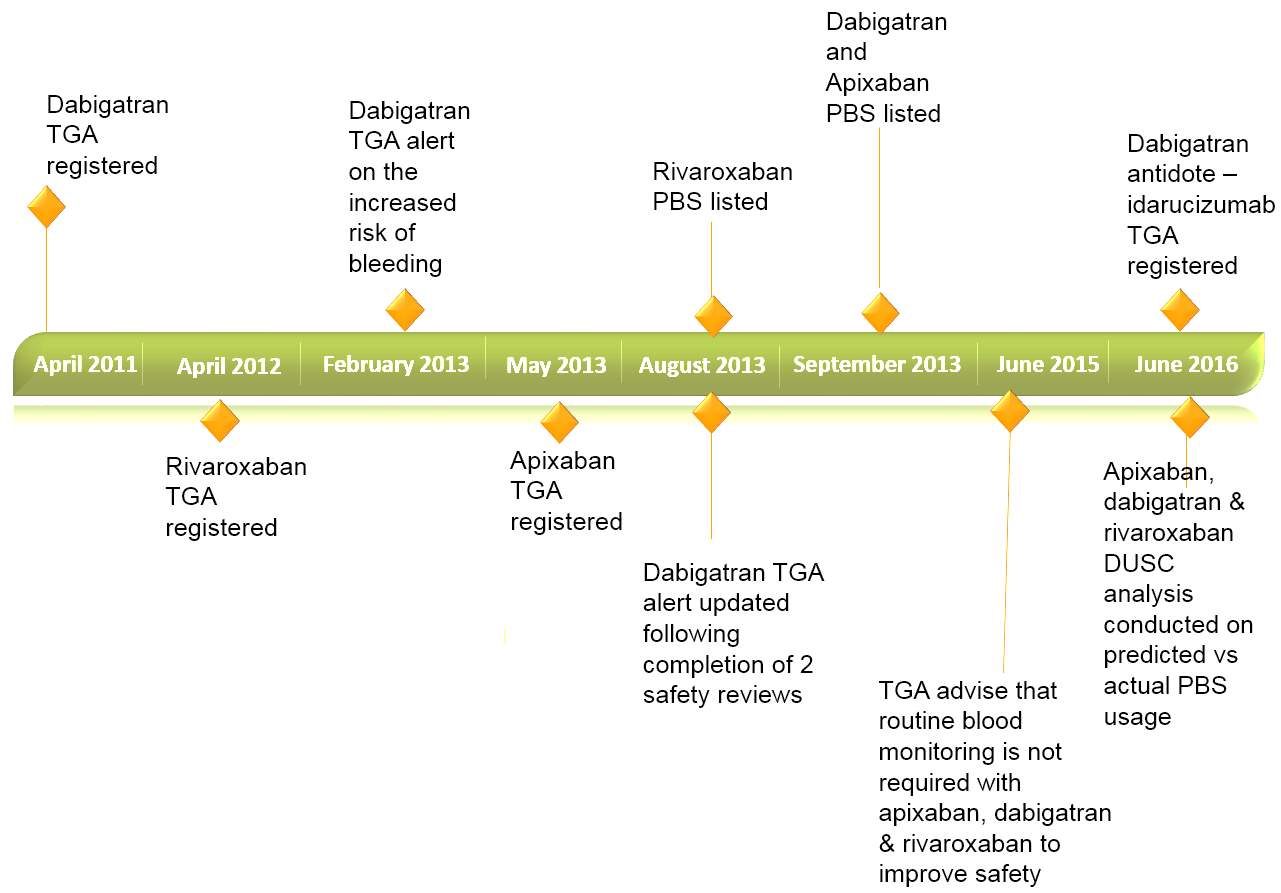
Figure 1. Non-vitamin K antagonist oral anticoagulant timeline for non-valvular atrial fibrillation.
a. This article avoids use of the term ‘NOACS: novel oral anticoagulants’ as this group of medicines is no longer ‘new’ due to their presence in clinical practice for more than a decade.
Risk of bleeding
As with any anticoagulants, safe and effective use of apixaban, dabigatran and rivaroxaban involves minimising the risk of thrombosis and bleeding.6 This involves carefully considering the suitability of one of these medicines for a particular indication, especially with regard to risk of bleeding, and the patient’s current stability on warfarin if switching from warfarin.7
Factors known to increase risk of bleeding
While the TGA stated that routine plasma monotoring is not required to manage bleeding in patients on one of these medicines,8 it is important to note that there are certain circumstances that may increase risk of bleeding.
The risk of bleeding is increased in patients who are on either apixaban, dabigatran or rivaroxaban and:5,7,9
- are aged 75 years or over
- have special haemorrhagic risks from diseases or recent medical procedures
- have moderate renal impairment (creatinine clearance 30–50mL/min)
- have anaemia
- are concurrently taking NSAIDs and/or antiplatelets (eg, clopidogrel or low-dose aspirin).
Non-vitamin K antagonist oral anticoagulants and low-dose aspirin therapy
Atrial fibrillation and coronary artery disease commonly occur together;10 thus patients are likely to be taking low-dose aspirin therapy in addition to an anticoagulant.
In patients with recent acute coronary syndrome, the addition of an anticoagulant to single antiplatelet therapy such as low-dose aspirin results in substantial increase in bleeding.11
A recent meta-analysis showed that in patients receiving single low-dose aspirin, additional treatment with an oral anticoagulant decreased the rate of major adverse cardiovascular events by 30% but clinically significantly increased the rate of bleeding events by 79%.11
The Australian National Heart
Foundation ACS guidelines recommend low-dose aspirin (100 mg–150 mg/day)
be stopped in patients in whom an anticoagulant is indicated,12
unless otherwise recommended by a cardiologist.
Non-vitamin K antagonist oral anticoagulants and NSAIDs
There have been reports of pharmacodynamic interactions between NSAIDs and this group of medicines that increase the risk of bleeding, as reflected in the pivotal trials of dabigatran13 and rivaroxaban.14
Despite this, unpublished data from the Australian Government Department of Veterans’ Affairs indicate that about 30% of veterans with atrial fibrillation taking aspirin, clopidogrel or warfarin monotherapy were co-prescribed concomitant NSAIDs or COX-2 inhibitors in 2012.9
More recent data from the Australian Government Department of Veterans’ Affairs indicate that NSAID use in 2013–14 was greater in patients taking apixaban, dabigatran or rivaroxaban compared with use in patients taking warfarin (8.7% vs 6.2%).4
These results are of concern, as they indicate that patients are taking an oral anticoagulant and an NSAID despite the increased risk of bleeding, particularly gastric bleeding.
Prescribers should carefully consider the concurrent use of non-vitamin K oral anticoagulant and NSAID and appropriately counsel their patients, as NSAIDs are easily accessible over the counter.
Other reasons that have contributed to bleeding incidents are:
- health professionals not recognising that apixaban, dabigatran and rivaroxaban are anticoagulants, thereby leading to concurrent prescribing of other anticoagulant medication
- inappropriate cessation of apixaban, dabigatran and rivaroxaban before surgical procedures.6
The NSW Clinical Excellence Commission NOAC Guidelines advise to use caution in the concomitant use of NSAIDs and apixaban, dabigatran or rivaroxaban, stating that antithrombotic interaction is similar to bleeding effects observed in antiplatelet and warfarin combinations.3
An antidote to reverse bleeding is now available for dabigatran. Idarucizumab (Praxbind) is TGA registered as:15
… a specific reversal agent for dabigatran and is indicated in patients treated with dabigatran etexilate (PRADAXA) when rapid reversal of the anticoagulant effects of dabigatran is required: – for emergency surgery / urgent procedures; – in life-threatening or uncontrolled bleeding.
Idarucizumab is available in hospitals and it not listed on the PBS. There are currently no antidotes for apixaban and rivaroxaban.
Practice points to avoid the risk of bleeding
- Advise patients to tell their doctor, dentist or pharmacist that they are taking an anticoagulant before starting or stopping any other medicine, herbal or over-the-counter products, particularly aspirin.
- Assess the need of aspirin in the patient’s management plan. Advise patients to avoid low-dose aspirin and other NSAIDs while taking an oral anticoagulant.
- Encourage patients to see a health professional if they have any unexplained bruising or bleeding, or pink, red or dark-brown urine.
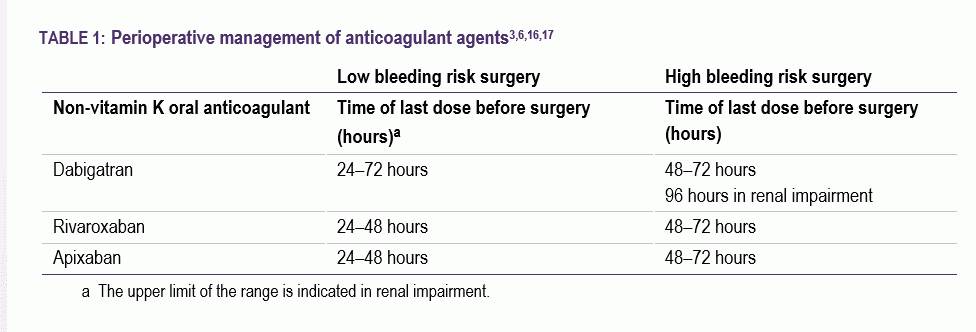
- Recognise that there are treatment cessation protocols (Table 1) if they undergo any surgical procedures.
- If switching patients from warfarin to apixaban, dabigatran or rivaroxaban, warfarin should be stopped, INR measured daily and the new medicine started when the patient’s INR is < 2.5.3
- Report adverse events and major bleeding events to the TGA.
References
- Australian Medicines Handbook 2015 (online). Adelaide: Australian Medicines Handbook Pty Ltd, 2015 January.
- Australian Government Department of Health, Drug utilisation sub-committee. Novel Oral Anticoagulants: Predicted vs actual analysis. June 2016. (Accessed 1 November 2016).
- Clinical Excellence Commission. Non-vitamin K Antagonist Oral Anticoagulant (NOAC) Guidelines. Sydney: 2016.(Accesssed 21 September 2016).
- Pratt NL, Ramsay EN, Caughey GE, et al. Uptake of novel oral anticoagulants in Australia. Med J Aust 2016;204:104-5 e1.
- Therapeutics Goods Administration. Apixaban (Eliquis), dabigatran (Pradaxa) and rivaroxaban (Xarelto): Information for health professionals. 2013. (Accessed 12 October 2016).
- Senior Pharmacist Medicines and Technology, Policy and Programs (MTPP). Safe prescribing of new oral anticoagulants: apixaban, rivaroxaban and dabigatran. SA Health Safety and Quality Strategic Governance Committee, 2015. (Accessed 5 October 2016.)
- Therapeutic Goods Administration. Dabigatran (Pradaxa) and risk of bleeding: Information for health professionals. 2013. (Accessed 5 October 2010).
- Therapeutics Goods Administration. New oral anticoagulants \u2013 apixaban (Eliquis), dabigatran (Pradaxa) and rivaroxaban (Xarelto). 2015. (Accessed 12 October 2016).
- Australian Government Department of Health. Review of Anticoagulation Therapies in Atrial Fibrillation. 2012. (Accessed 28 September).
- Thompson PL, Verheugt FW. Managing antithrombotic therapy in patients with both atrial fibrillation and coronary heart disease. Clin Ther 2014;36:1176\u201381.
- Oldgren J, Wallentin L, Alexander JH, et al. New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis. Eur Heart J 2013;34:1670\u201380.
- Chew DP, Scott IA, Cullen L, et al. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Australian Clinical Guidelines for the Management of Acute Coronary Syndromes 2016. Heart Lung Circ 2016;25:895\u2013951.
- Boehringer Ingelheim. Product Information: Pradaxa (dabigatran etexilate). 2012. (Accessed 18 April 2012).
- Bayer Australia LTD. Product Information: Xarelto. 2016. (Accessed 12 October 2016).
- Therapeutics Goods Administration. ARTG summary for Praxbind idarucizumab (rch) 50 mg/mL solution for injection/infusion vial. 2016. (Accessed 12 October 2016).
- Rahman A, Latona J. New oral anticoagulants and perioperative management of anticoagulant/antiplatelet agents. Aust Fam Physician 2014;43:861\u20136.
- Thrombosis Canada. Peri-operative management of patients who are receiving a new oral anticoagulant. 2013. (Accessed 14 September 2016).
