A new PBS listing for second-line treatment of chronic symptomatic gout associated with hyperuricaemia
Key points
-
Febuxostat inhibits uric acid production
It is a non-purine selective inhibitor of xanthine oxidase that decreases serum uric acid level by inhibiting the formation of uric acid from xanthine. -
PBS listed for second-line treatment
Only for people for whom allopurinol is contraindicated, or who are hypersensitive to, or intolerant of, allopurinol. -
Dose titration is recommended
The recommended starting dose is 40 mg once daily (achieved by halving one 80 mg tablet). If serum uric acid levels remain > 0.36 mmol/L after 2–4 weeks, increase dose to 80 mg once daily. -
There may be an initial increase in gout flares
Consider concurrent prophylaxis for at least 6 months with low-dose colchicine or an NSAID.
Evidence snapshot
What is known about this drug
Febuxostat is a non-purine selective inhibitor of xanthine oxidase. It is used for the treatment of chronic symptomatic hyperuricaemia in adults with gout.
In trials, febuxostat 80 mg/day was shown to reduce and maintain serum uric acid levels below the guideline target of 0.36 mmol/L in most patients (about 70%).
After starting xanthine oxidase inhibitors, the incidence of gout flares increases due to lowering of serum uric acid levels resulting in the release of uric acid from tissue deposits. Avoid starting febuxostat during flares but do not stop use after starting even if flares occur.
In trials the most common adverse reaction leading to discontinuation from therapy was liver function abnormalities.
In view of the numerical increase of cardiovascular events in trials, febuxostat is not recommended in patients with ischaemic heart disease or congestive heart failure.
Avoid febuxostat in people taking mercaptopurine and/or azathioprine, as concomitant use may substantially increase plasma concentrations of these drugs, leading to severe toxicity.
Areas of uncertainty
The results of the pivotal clinical trials should be interpreted with caution, as the design may not reflect best clinical practice.
While there is a clinical need for an alternative to allopurinol, the evidence for superiority of febuxostat over allopurinol is not adequately supported by current trial data. The pivotal trials did not titrate allopurinol to its optimal dose according to the effect on serum urate, causing uncertainty regarding the comparative effectiveness of febuxostat and allopurinol.
Furthermore, there were no statistically significant improvements in the number of tophi and percentage reduction of tophus area from baseline with febuxostat compared with allopurinol.
There is also inadequate clinical evidence to establish efficacy and safety of febuxostat in patients who are poor responders to maximally tolerated doses of allopurinol, and in those with hypersensitivity to allopurinol.
Some patients who reported serious hypersensitivity reactions to febuxostat also reported previous hypersensitivity to allopurinol. Therefore use caution when prescribing for Han-Chinese people; they are known to be at high risk of allopurinol hypersensitivity but there is lack of evidence on whether they also have elevated risk of febuxostat hypersensitivity.
There is uncertainty about the potential long-term risk in cardiovascular safety (cardiovascular death, non- fatal MI, and non-fatal stroke). The European Medicines Agency has requested a post-licensing cardiovascular safety study of febuxostat versus allopurinol – the FAST study – which is underway, with a minimum of 3 years' follow-up.
What does NPS MedicineWise say?
Trials have demonstrated the efficacy of febuxostat in reducing and maintaining serum uric acid at target levels. However, febuxostat has not demonstrated superior effectiveness over allopurinol.
Consider febuxostat for second-line treatment in patients for whom allopurinol is contraindicated, or who are intolerant of allopurinol.
In current clinical practice, allopurinol is often used at suboptimal doses, leading to treatment failure. Ensure allopurinol dosage has been titrated according to serum urate level (< 0.36 mmol/L).
Febuxostat is not PBS listed for people in whom the maximum approved dose of allopurinol (900 mg/day) has failed.
Due to lack of long-term cardiovascular safety data, prescribing febuxostat in patients with ischaemic heart disease or congestive heart failure is not recommended.
Avoid prescribing febuxostat with mercaptopurine and/or azathioprine, as concomitant use may lead to elevated serum levels of these drugs, causing bone marrow depression.
Quality of the evidence
See Quality of the evidence section below.
PBS listing
Authority required
PBS indication is chronic gout.
The condition must be either chronic gouty arthritis or chronic tophaceous gout.
The patient must satisfy one of the following clinical criteria:
- patient must have a medical contraindication to allopurinol; OR
- patient must have a documented history of allopurinol hypersensitivity syndrome; OR
- patient must have an intolerance to allopurinol, necessitating permanent treatment discontinuation.
May be prescribed by nurse practitioners (shared care model)
Authorised nurse practitioners may prescribe this medicine as part of a formal care plan with a medical practitioner. See the PBS website for more information on nurse practitioner PBS prescribing.
What is it?
Febuxostat is a non-purine selective inhibitor of xanthine oxidase that decreases serum uric acid (sUA) level by inhibiting the formation of uric acid from xanthine.1 It comes in tablet form with a starting dose of half an 80 mg tablet taken once daily.1
It is approved by the Therapeutic Goods Administration as an S4 drug and indicated for the treatment of chronic symptomatic hyperuricaemia when urate deposition has already occurred in adults with gout.1
Who is it for?
Febuxostat may be an option for people who have chronic gouty arthritis or in people who present with tophi (uric acid crystal) depositions.
Febuxostat is PBS listed for patients who:
- have either a medical contraindication, or a history of hypersensitivity, to allopurinol, or
- are unable to continue treatment with allopurinol due to intolerance.2
Due to lack of long-term cardiovascular safety data and an increased number of cardiovascular events compared with allopurinol in the trials,3-5 febuxostat is not recommended in patients with ischaemic heart disease or congestive heart failure. It is also not recommended in people taking mercaptopurine and/or azathioprine.
Febuxostat is eliminated by both hepatic and renal pathways; use with caution in patients with severe renal or hepatic impairment, as there are insufficient data on efficacy and safety in this patient population.1
Where does it fit?
The goal in treating chronic gout is to dissolve tophi and prevent progressive joint damage,6, 7 which may be achieved by aggressive lowering of plasma urate levels.6
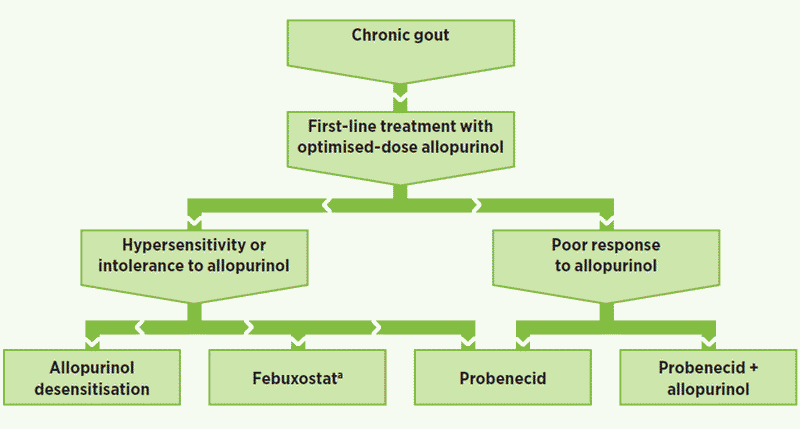
Not stated in Therapeutic Guidelines; positioned in accordance with Pharmaceutical Benefits Advisory Committee recommendations
(Diagram adapted from eTG Rheumatology, 20103)
Both febuxostat and probenecid are available as alternatives to allopurinol in people who are intolerant of, or have hypersensitivity to, allopurinol. However, currently only probenecid is available on the PBS for those with a poor response to allopurinol.
The Pharmaceutical Benefits Advisory Committee (PBAC) declined to allow poor response to allopurinol as an eligibility criterion for PBS-subsidised febuxostat due to lack of clinical evidence in this patient population.2
How does it compare?
The pivotal trials – FACT,3 APEX4 and CONFIRMS5 – were considered to support efficacy for purposes of regulatory approval.1, 8 Supporting evidence of efficacy included two open-label extension trials – FOCUS9 and EXCEL.10
The FACT and APEX trials assessed the efficacy and safety of febuxostat 80 mg, 120 mg,3, 4 and the CONFIRMS study assessed 40 mg and 80 mg, compared with 300 mg allopurinol. An allopurinol dose of 100 mg was used in patients with renal impairment in the FACT and APEX studies, and 200 mg in the CONFIRMS study.3-5
The primary efficacy endpoint in both FACT3 and APEX4 studies was the proportion of patients whose last 3-monthly serum uric acid levels were < 0.357 mmol/L.1
Febuxostat and allopurinol superior to placebo
The APEX study demonstrated a significant difference in the proportion of patients who achieved target sUA levels compared with placebo (febuxostat 80 mg: 48%, 120 mg: 65%, allopurinol 300 mg: 22%, placebo: 0%).
Although the APEX study was only 28 weeks in duration, long-term studies (CONFIRMS and FOCUS) demonstrated the durability of the response with most patients taking the 80 mg dose.4, 8
Febuxostat not superior to allopurinol
The pivotal trials assessed non-inferiority to allopurinol based on the proportion of patients who achieved target sUA levels.
The FACT study reported a 32% difference and the APEX study reported a 26% difference in the proportion of patients who achieved the target sUA levels in the febuxostat 80 mg arm compared with the allopurinol 300 mg arm.11
The CONFIRMS study reported comparable efficacy of febuxostat 40 mg compared with allopurinol 300 mg (febuxostat 40 mg: 45%, 80 mg: 67%, allopurinol 300 mg: 42%).6
However, these results should be interpreted with caution, as the study designs did not reflect best clinical practice because allopurinol dosage was not titrated to optimal effect.
Guidelines recommend people with normal renal function start allopurinol at 100 mg/day orally for the first month, then increase the dose by 50–100 mg every 4 weeks and titrate according to serum uric acid levels and response. The maximum tolerated dose of allopurinol (up to 900 mg/day) may be used to achieve sUA target levels.7
Follow current recommendations on allopurinol dose selection and titration in people with renal impairment.7
Reduced urate level not a surrogate for patient-relevant outcomes
The primary endpoint of the pivotal trials was the proportion of patients who achieved a reduction in sUA level to < 0.36 mmol/L.3-5, 12
This efficacy measure is a common target outcome in urate-lowering therapy studies and is recommended by Australian Medicines Handbook and the American College of Rheumatologists as a guide for urate-lowering therapy dosing.7, 13
For purposes of establishing efficacy, the TGA was satisfied that the primary outcome in the pivotal trials was an appropriate efficacy measure for determining the utility of a therapy in treating symptomatic hyperuricaemia.8
However, in determining the comparative effectiveness of febuxostat and allopurinol, the PBAC did not agree with the assumption that attaining the biological sUA target (< 0.36 mmol/L) would closely correlate with clinically meaningful outcomes such as flare reduction, tophi resolution or improved-health-related quality of life.11
Nonetheless, the PBAC recognised the clinical need for an alternative treatment in patients who are intolerant of allopurinol and thus recommended the PBS listing of febuxostat in this patient population, in which it is likely to be cost-effective compared with probenecid.2
Safety issues
A total of 4072 subjects received at least one dose of febuxostat in the studies presented for regulatory approval.6 The most frequent adverse events, with a frequency of ≥ 1% in febuxostat treatment arms, included:1
- skin reactions
- abnormal liver tests (frequency of ≥ 3% in the pivotal trials)
- non-infective diarrhoea
- headache and nausea
- increased risk of gout flares when starting therapy (31% for febuxostat 40 mg and 43% for 80 mg1), with the need for co-administration of gout prophylaxis treatment (NSAID or low-dose colchicine) for at least 6 months.3, 4, 8
The most common adverse reaction leading to discontinuation from therapy was liver function abnormality (1.8% of the febuxostat 40 mg arm, 1.2% of the febuxostat 80 mg arm, and 0.9% of allopurinol-treated subjects).1
Consider liver tests before starting febuxostat. Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
In this clinical context, if the patient is found to have abnormal liver tests ( ALT greater than three times the upper limit of the reference range), stop febuxostat treatment and investigate the probable cause.1
The pivotal and supplementary evidence for regulatory approval also showed a numerical increase in investigator- reported cardiovascular events (cardiovascular death, non-fatal MI, and non-fatal stroke) with febuxostat compared with allopurinol.1
The increase was not statistically significant and no causal relationship was established. However, the European Medicines Agency has requested a post-licensing cardiovascular study of febuxostat versus allopurinol – the FAST study – as part of the febuxostat pharmacovigilance plan.14
The FAST study is a prospective, randomised, open-label blinded-endpoint trial currently underway, with a minimum of 3 years' follow-up and due to end in September 2016.15
Dose strengths approved in Australia
Only the 80 mg tablet dose strength is TGA approved in Australia.
However, on the basis of evidence from the CONFIRMS trial, which demonstrated non-inferiority of the 40 mg dose to allopurinol 300 mg/day, 6, 7 and the cardiovascular safety concerns (see Safety issues), the TGA has recommended that the lowest effective dose be used until further data become available on cardiovascular outcomes.
This is a starting dose of 40 mg, with the possibility of titration to a maximum dose of 80 mg.1, 8 Given that only the 80 mg dose is approved in Australia, patients will be required to split the scored tablet in half to achieve a 40 mg starting dose.
For information about reporting adverse reactions to the TGA, or to report suspected adverse reactions online, see the TGA website.
Reason for PBS listing
The PBAC considered there were insufficient data to support a claim of superior efficacy over allopurinol for patient-relevant outcomes such as reduced incidence of acute gout attacks or tophi or improved quality of life, and therefore did not recommend listing for first-line use.11
The PBAC recommended listing febuxostat as second-line therapy on the basis of:
- a clinical need for an alternative to probenecid in patients in whom allopurinol is contraindicated or not tolerated
- febuxostat being likely to represent, at the price proposed by the sponsor, a cost-effective treatment compared with probenecid in a targeted, second-line treatment patient population.2
With respect to safety, the PBAC considered that the comparative harms of febuxostat were no worse than those of allopurinol, probenecid and allopurinol + probenecid.2
Dosing recommendations
The recommended starting dose of febuxostat is 40 mg, taken by mouth once daily with or without food at around the same time every day.1
As only the 80 mg strength is available in Australia as a scored tablet, advise patients to break the tablet in half to obtain a 40 mg dose. Patients can either use their fingers to break the tablet, or if this proves to be too difficult, advise patients to use a pill cutter (available at any pharmacy).
If serum uric acid is > 0.36 mmol/L after 2–4 weeks, increase the dose to 80 mg once daily.1
Note that febuxostat dose adjustments are not necessary in the elderly and patients with mild to moderate renal impairment.
When starting treatment, advise patients to continue taking febuxostat even if they experience gout flares. Consider concurrent prophylaxis for at least 6 months with an NSAID or low-dose colchicine (maximum 0.5 mg once or twice daily), according to response and gastrointestinal symptoms.1 Reduce colchicine dose in renal impairment.
Febuxostat is eliminated by both hepatic and renal pathways; use with caution in patients with severe renal or hepatic impairment, as there are insufficient data on efficacy and safety in this patient population.1
Medicine interactions
Febuxostat use may substantially increase plasma concentrations of azathioprine and/or mercaptopurine through its action on xanthine oxidase, leading to increased risk of bone marrow depression from azathioprine and/or mercaptopurine toxicity.1
Avoid these combinations if possible or reduce the dose of these drugs and monitor for haematological effects (including changes to white blood cell counts, haemoglobin, platelet count and bleeding).1
Information for patients
- Before starting febuxostat, advise patients that a liver function blood test is recommended and repeated during therapy if symptoms indicative of liver injury are experienced (such as fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice).
-
To obtain the starting dose of 40 mg, advise patients to hold the tablet between their thumbs and index fingers close to the score line and apply pressure to snap the tablet in half.
16
- If this is too difficult to achieve, recommend the use of a pill cutter. Advise patients to speak to their pharmacist for further instructions on halving the tablet using a pill cutter.
- Discuss with your patient that it may take several months before febuxostat begins to prevent gout attacks. Explain that there may be an increase in the number of gout attacks (flares) for at least the first 6 months of treatment.
- Educate patients to continue using febuxostat despite gout flares and reinforce the importance of treatment continuation. Advise patients that eventually the flares will become less frequent and less intense.
- To help alleviate pain during flares or minimise the risk, explain to patients that they will be prescribed an NSAID or low-dose colchicine for at least the first 6 months of treatment.16, 17
- Advise patients to report any signs or symptoms of cardiovascular events or a stroke (eg, chest pain, shortness of breath, or neurological symptoms).
- Discuss the Adenuric Consumer Medicine Information (CMI) leaflet with the patient.
Quality of the evidence
For selected RADAR reviews, NPS MedicineWise summarises the evidence used in developing our conclusions about a new medicine's efficacy and safety using the GRADE criteria for assessing quality of the evidence.17, 18
Risk of bias
Is there bias in the study design and approach?
There is a very high risk of bias in the study design of the pivotal trials.
None of the trials allowed an optimised allopurinol dose of up to 900 mg/day, which is considered as current best clinical practice.
This greatly favours the febuxostat treatment arm when making conclusions on comparative effectiveness.
Furthermore, in the FACT study and the FOCUS extension studies, 50% of patients did not complete the study. The APEX study reported that 28% of participants failed to complete the study, of whom 23% discontinued because of adverse events.
The pivotal studies were randomised double-blinded trials with appropriate and internationally approved primary and secondary outcome measures. The extension trials, however, were open label, increasing the risk of bias. The EXCEL study had high crossover of patients from allopurinol to febuxostat (~60%).
Some authors of the trials were employees of the pharmaceutical company manufacturing febuxostat, and all trials were sponsored by the pharmaceutical company, which poses a potential conflict of interest as a source of bias.
Overall the trials did not have adequate design to properly evaluate the comparative effectiveness of febuxostat and allopurinol.
Inconsistency
Are the data consistent between trials?
All trials used the same primary outcome of a target sUA level of < 0.36 mmol/L.
All head-to-head trials used common treatment arms of febuxostat, allopurinol and/or placebo with standard dosing of 80 mg febuxostat (or 40 mg and 120 mg in some trials) and 300 mg allopurinol.
Treatment effects (effective reduction of sUA) were consistent between the pivotal and extension trials. The trend of a higher proportion of patients achieving the target sUA levels with increasing dose of febuxostat was seen across all trials (except for the FOCUS study).
The nature of the adverse events were common across all trials. Additionally, a similar trend of increasing drop- out rates due to adverse events associated with higher doses was seen across trials.
In general, treatment effect across studies is comparable and the population, intervention and outcomes were consistent across trials.
Indirectness
Are the trial data applicable to the wider population that will be prescribed this medicine?
Given that the study design did not allow appropriate comparison of febuxostat to allopurinol, as the optimal allopurinol dose was not used, there are limitations in applying trial results to the Australian population.
There is also a lack of data in the effectiveness of febuxostat in patients who are insufficient or poor responders to allopurinol, or patients who are hypersensitive to allopurinol.
There are further limitations in assessing long-term adverse events from current studies, as the maximum follow-up was 5 years, and the FOCUS study had low patient numbers.
Given that febuxostat treatment is long term, studies beyond 5 years in a higher patient sample size are required to adequately assess safety and apply results to the wider population.
Additionally, the trials have excluded patients with severe renal and hepatic impairment.
While the populations examined in the phase III studies are similar in demographics and have similar background treatment to patients who would be treated in Australian clinical practice, there are limitations in applying the data to the broader Australian population.
Imprecision
Are the confidence intervals around the estimate of effect wide?
Confidence intervals (CI) were only analysed in the CONFIRMS, FACT and APEX studies to determine non-inferiority and superiority to allopurinol.
According to the trials, non-inferiority to allopurinol was consistently demonstrated using the pre-specified criteria of the lower 97.5% CI being > –10% in FACT and APEX.
The CONFIRMS study claimed non-inferiority of febuxostat 40 mg to allopurinol 300 mg, but the difference in response rates between the two groups was 3.1% (95% CI: –1.9% to 8.1%) and not significant.7
The results of the FOCUS study may be imprecise due to small patient numbers and high discontinuation rates, so these results should be treated with caution.
Overall, the pivotal trials had sufficient patient numbers with acceptable CI ranges.
Publication bias
Is there evidence that trials have been selectively published?
Confidence intervals from the APEX study were not reported in Schumacher et al 2008.5
Furthermore, some of the authors were employees of the manufacturing pharmaceutical company and all trials were sponsored by the pharmaceutical company.
The above factors may contribute to an overestimate of the treatment effect due to the potential risk of publication bias.
Conclusion
The quality of evidence to support the efficacy and safety of febuxostat is moderate.
There is moderate risk of bias across the pivotal trials; trials were generally of adequate design to determine the efficacy of febuxostat alone, but not robust in evaluating the comparative effectiveness of febuxostat and allopurinol.
The results of the primary efficacy outcome are valid, and consistent treatment effects were seen throughout the phase III and extension studies. However, caution should be used in comparing febuxostat with allopurinol in terms of reducing sUA levels and clinical outcomes, as the results may not be applicable to the Australian population, in whom the optimal dose of allopurinol may be titrated to 900 mg/day.
References
- A. Menarini Australia Pty Ltd. Adenuric (febuxostat) Product Information. 2015. [TGA] (accessed 5 June 2015).
- Australian Government Department of Health. Public Summary Document. Febuxostat. Adenuric. 2015. [PBS]
- Becker MA, Schumacher HR, Jr., Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450\u201361. [PubMed]
- Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 2008;59:1540\u20138. [PubMed]
- Becker M, Schumacher HR Jr, Espinoza L. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: The CONFIRMS trial. Arthritis Res Ther 2010;12. [Arthritis Research]
- Rheumatology Expert Group. Therapeutic Guidelines: Rheumatology. Version\u00a02. Melbourne: Therapeutic Guidelines Limited, 2010. [TG online] (accessed 22 May 2015).
- Rossi S (ed). Australian Medicines Handbook. Adelaide: Australian Medicines Handbook Pty Ltd, 2015.
- Therapeutic Goods Administration. Australian Public Assessment Report for Febuxostat (FBX) 2015. [TGA]
- Schumacher HR Jr, Becker M, Lloyd E, et al. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology 2009;48:188\u201394.
- Becker M, Schumacher HR Jr, MacDonald P, et al. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol 2009;36:1273\u201382. [The Journal of Rheumatology]
- Australian Government Department of Health, Pharmaceutical Benefits Scheme. Public Summary Document. FEBUXOSTAT, tablet, 80\u00a0mg, and 120\u00a0mg, Adenuric. Canberra: PBS, 2014. [PBS (online PDF)] (accessed 13 May 2015).
- Becker MA, et al. Arthritis Res Ther 2010;12:R63.
- Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology Guidelines for Management of Gout. Part 1: Systematic Nonpharmacological and Pharmacological Therapeutic Approaches to Hyperuricemia. Arthritis Care Res 2012;64:1431\u201346. [American College of Rheumatology.
- European Medicines Agency. CHMP Assessment Report for Adenuric. London: EMA, 2008. [EMA (online PDF)]
- MacDonald TM, Ford I, Nuki G, et al. Protocol of the Febuxostat versus Allopurinol Streamlined Trial (FAST): a large prospective, randomised, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia. BMJ Open 2014;4:e005354. [PubMed] .
- A. Menarini Australia Pty Ltd. Adenuric 80\u00a0mg Tablets Consumer Medicine Information 2014. [TGA]
- Medline Plus. Febuxostat. 2009. [MedlinePlus] .
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924\u20136. [PubMed]
