Key points
- For all people with CKD, prescribe an ACE inhibitor or ARB, up-titrated to the maximum tolerated dose.
- Add an SGLT2 inhibitor (dapagliflozin), and blood pressure-lowering, lipid-lowering and glucose-lowering medicines, as determined by clinical factors such as whether the person is meeting a therapeutic target.
- The order in which the above medicines are added is guided by compelling indications such as albuminuria levels, the underlying cause and any comorbidities.
- A person-centred approach to prescribing these medicines includes building your relationship with the patient over the long term and finding ways to help the patient feel they are in control of their health.
- CKD shares many treatment goals and management strategies with CVD and type 2 diabetes. Use a ‘whole of person’ approach to manage these chronic conditions in conjunction.
- Adjust doses of medicines cleared by the kidneys according to kidney function to reduce risk of adverse effects and avoid nephrotoxic medicines where possible.
A practical guide to medicines for chronic kidney disease
Australian and international guidance is clear on medicines for chronic kidney disease (CKD) that slow CKD progression and reduce cardiovascular (CV) risk.
All people with CKD should first be prescribed an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB).1,2
Other medicines can then be added as needed to slow CKD progression and reduce CV risk, including:1-7
- sodium–glucose co-transporter-2 (SGLT2) inhibitors (dapagliflozin) as add-on therapy to an ACE inhibitor or ARB
- blood pressure-lowering medicines to treat hypertension
- lipid-lowering medicines for elevated absolute CV risk
- glucose-lowering medicines for elevated glycaemic levels in type 2 diabetes.
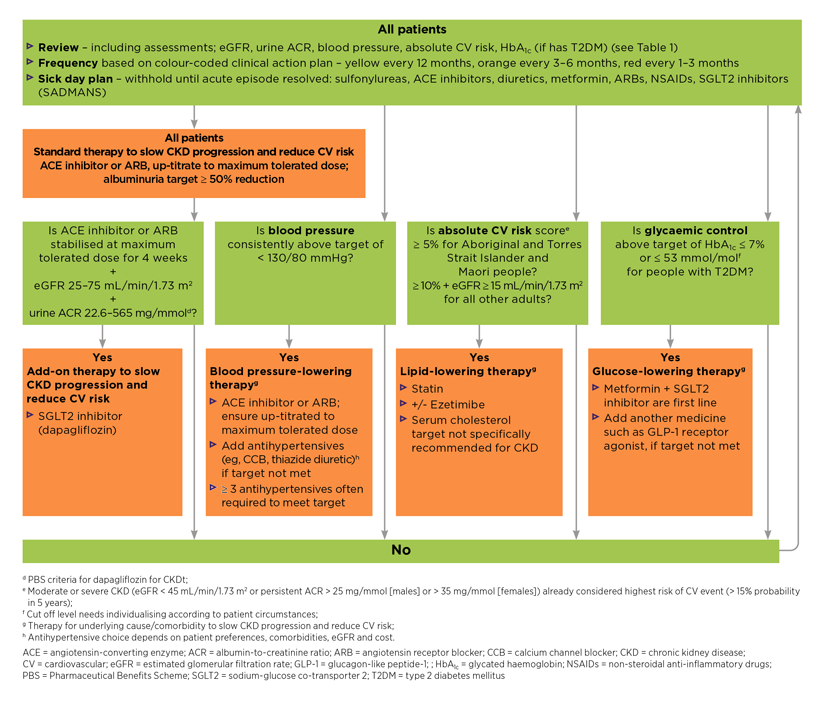
This article provides a practical guide on when and how to prescribe the above medicines for people with CKD. It includes an algorithm (see Figure 2) and follows the case study of a patient named Ken.
Download and print
Case study: Ken

Ken has been newly diagnosed with CKD.
He is a 64-year-old male, married, with two adult-aged children and is a taxi owner/driver. He hasn’t smoked for 20 years.
Ken’s medical history includes hypertension (at last check it was 145/90 mmHg) and dyslipidaemia, both diagnosed 5 years ago.
His medicines include perindopril erbumine 2 mg daily and atorvastatin 40 mg daily.
See NPS MedicineWise CKD – Integrating kidney health into patient care for how to screen and make a diagnosis of CKD.
Management based on risk level
The management for each person with CKD is guided by where that person’s estimated GFR (eGFR) and ACR results place them on a colour-coded staging table.1 See Figure 1.

Ken has an eGFR of 56 mL/min/1.73 m2, which is Stage 3a CKD.
His urine ACR is 24 mg/mmol, which is microalbuminuria.
This places Ken at a high-risk level for progression of CKD and developing cardiovascular disease (CVD).
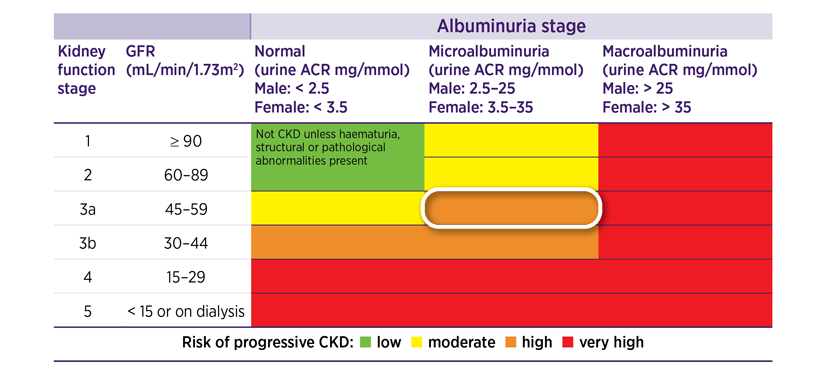
Figure 1: Staging of CKD and colour-coded risk levels that guide clinical action plans for managementa
aRisk level colour codes: green = low risk, yellow = moderate risk, orange = high risk and red = very high risk.1,9
Source: KHA-CARI Guideline: Early chronic kidney disease: Detection, prevention and management. Johnson DW, Atai E, Chan M, et al. Nephrology 2013;18:340–50. Wiley Online Library.
Clinical action plans for management
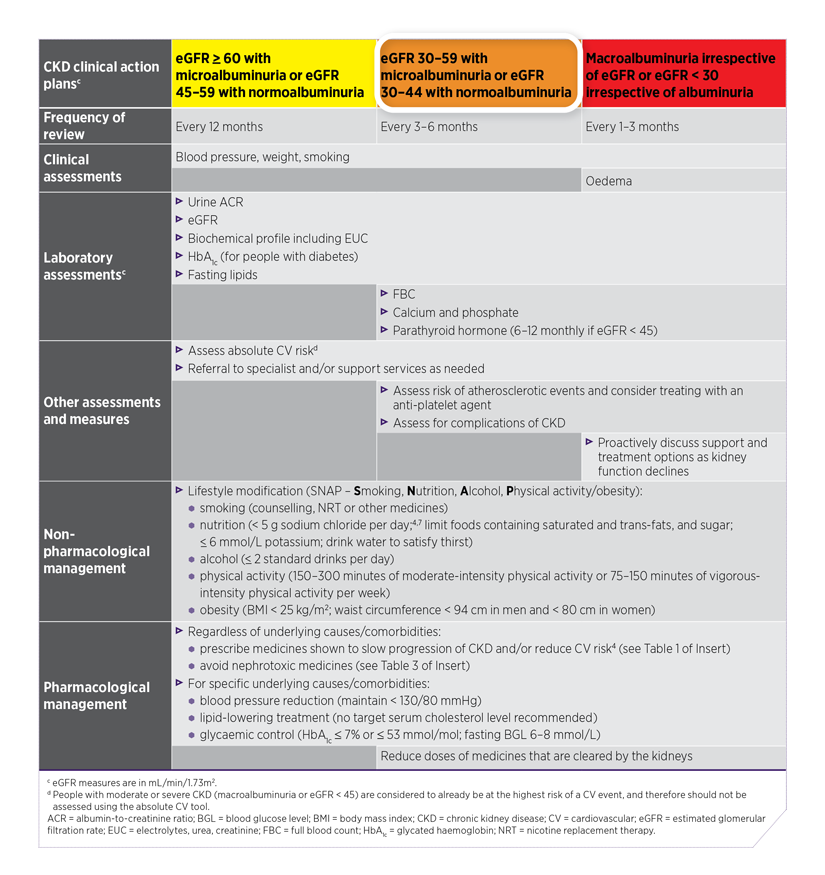
The risk levels in the colour-coded CKD staging table correspond to colour-coded clinical action plans presented in the Kidney Health Australia 2020 CKD Management in Primary Care 4th edition handbook.1 See Table 1 below for an adapted version of these clinical action plans.
Each clinical action plan includes a set of clinical and laboratory assessments and measures, management goals, and non-pharmacological management and pharmacological management recommendations, including medicines to slow CKD progression and reduce CV risk.1

Ken’s risk level places him in the orange clinical action plan, which recommends a review of his CKD every 3–6 months.
Table 1: Assessment and management for people with CKD based on colour-coded clinical action plans1
Underlying cause

Ken was diagnosed with hypertension and dyslipidaemia 5 years ago.
Investigations performed as part of Ken’s newly diagnosed CKD have determined that the underlying cause of the CKD is hypertension.
See NPS MedicineWise CKD – Integrating kidney health into patient care for the recommended investigations for newly diagnosed CKD.
The appropriate recording of a CKD diagnosis in Ken’s patient record includes his eGFR stage, albuminuria level and underlying cause:1
Stage 3a CKD with microalbuminuria due to hypertension.
Recommended medicines and reviews for Ken to slow CKD progression and reduce CV risk
An algorithm based on the Australian and international guidance presents the recommended medicines that slow CKD progression and reduce CV risk for people with CKD. See Figure 2 below.

The medicines and reviews recommended for Ken based on this algorithm are as follows:
- An ACE inhibitor or ARB; standard therapy for all people with CKD.
- Blood pressure-lowering therapy to treat hypertension, the underlying cause of his CKD.
- Review the need for add-on therapy to an ACE inhibitor or ARB, with an SGLT2 inhibitor (dapagliflozin).
- Review Ken’s current lipid-lowering therapy for his dyslipidaemia, including an assessment of his absolute CV risk and eGFR level.
Integrating CKD management into patient care
CKD rarely occurs in isolation. Of the estimated one in 10 adults with probable CKD in Australia, 35% have CVD and CKD, 6% have type 2 diabetes and CKD, and 11% have all three.1,13
CKD shares many treatment goals and management strategies with CVD and type 2 diabetes. Taking a ‘whole of person’ approach and managing these chronic conditions in conjunction with one another can lead to improved patient outcomes.1

Dr Tim Senior, GP at Tharawal Aboriginal Corporation, clinical senior lecturer at the University of Western Sydney Medical School and CKD expert in general practice
“There is an overlap between CKD, cardiovascular disease and diabetes with regards to management.
We as GPs are already doing much of what is needed for CKD in our management of these other conditions. You don’t need to do much more to manage CKD.”

A GP’s strategy to support confidence
Dr Caitlin Sum, Adelaide GP and member of the Kidney Health Australia Primary Care Educational Advisory committee.
“Prescribing medicines that slow CKD progression and reduce cardiovascular risk for people with newly diagnosed CKD can be done over several consultations. In most cases there is ample time to optimise a patient’s management.
“This enables me to start with the medicines I’m confident prescribing. For many GPs this will be an ACE inhibitor or an ARB. For medicines I’m less familiar with, I develop a list of priorities about doses that need to be changed or medicines started.
“I know I have time to upskill or refresh my knowledge when required before the next appointment by reading the guidance or contacting my local hospital renal service or a private nephrologist. So when the patient comes back, I can be confident with prescribing them.”
Person-centred approach to medicines for CKD

Graeme Turner, nurse practitioner in the Northern NSW Local Health District and co-author of the 2013 KHA-CARI Guideline: Early chronic kidney disease: Detection, prevention and management
“The main task with person-centred care is to spark interest and help people with CKD be motivated to get involved in their care from the start.
“This involves finding ways to give the person a feeling they have some control over their health, rather than the health professional trying to control their health.
“CKD has its challenges, particularly because it is usually asymptomatic. I find using the colour-coded staging table can be helpful. I explain that taking their medicines and making lifestyle changes can reduce their ACR number, which means they’re slowing the progression of disease. You can use reducing their blood pressure number as a motivator in the same way.”
How to decide what order to prescribe medicines?
All people with CKD should first be prescribed an ACE inhibitor or ARB, up-titrated to the maximum dose.
What is the next step? What guidance should GPs follow to decide the order of medicines?
Dr Caitlin Sum
“My approach is to first pick the low-hanging fruit. I look at what the patient is already taking and prioritise a dose that needs up-titrating. Then I prioritise treating the underlying cause and any comorbidities.
“Patients often need to take multiple medicines, so you need to have their agreement to come back over a number of appointments to monitor them and see what works. You don’t have to do everything at once, your focus is on the long term and building your relationship with the patient.”

Compelling indications that guide prioritisation
Professor Ivor Katz, nephrologist at St George Hospital in Sydney and member of the Kidney Health Australia Primary Care Educational Advisory committee.
“Compelling indications guide my decisions about which medicines to prescribe. The most compelling are usually targeting the albuminuria level, because it is just such a significant factor for cardiovascular risk, but I still target… hypertension and diabetes and comorbidities like dyslipidaemia and obesity with support from GPs, and for me I will of course also focus on the underlying cause of the CKD if it's not hypertension or diabetes.
“For people with high albuminuria levels, my priority is to up-titrate the ACE inhibitor or ARB to maximum tolerated dose, then I add on a SGLT2 inhibitor.
“For lower albuminuria levels I’ll increase the ACE inhibitor or ARB dose once, but then look at increasing another medicine they’re already taking or start a new medicine for their existing hypertension or diabetes and lastly focus on a specific underlying cause. Then I’ll go back to up-titrating the ACE inhibitor or ARB."
ACE inhibitor (or ARB) – standard therapy to slow CKD progression and reduce CV risk

The first step recommended for Ken is to up-titrate the currently prescribed low dose of ACE inhibitor, perindopril erbumine 2 mg daily, to maximum tolerated dose to:1
- reduce his albuminuria levels, and
- treat his hypertension (see Blood pressure-lowering therapy section for more details).
Target
The recommended therapeutic target for albuminuria is ≥ 50% reduction.1,12 This recommendation is based on expert opinion and trial evidence of relative risk reduction in kidney failure and CV events due to reduction in albuminuria.
The target maximum dose for perindopril erbumine is 8 mg daily.7 Start and target maximum doses for ACE inhibitors and ARBs for people with CKD are found in the KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease (see Page S33, Figure 3).
Guidance
Professor Ivor Katz
“The dose increase for up-titration of an ACE inhibitor or ARB should be done every 4 to 6 weeks. Because it can take time for the albuminuria to decrease, I measure the ACR around three times a year for most patients.”
When starting an ACE inhibitor or ARB, or at each dose increase:1
- monitor eGFR, potassium and blood pressure
- a reversible reduction in eGFR is expected at initiation
- continue if eGFR reduction < 25%
- decline in eGFR > 25% requires assessment to determine if cessation and/or referral to a nephrologist is required.
ACE inhibitors and ARBs should be temporarily stopped during periods of illness or when dehydrated. They should be recommenced when the acute episode has resolved.1,11
Blood pressure-lowering therapy

Up-titrating the ACE inhibitor to the maximum tolerated dose is expected, in addition to reducing albuminuria, to decrease Ken’s blood pressure. It was 145/90 mmHg at the last check.
If Ken's blood pressure remains above target, further blood pressure-lowering medicines may need to be added. Often, three or more blood pressure-lowering medicines are needed for most people with CKD to adequately meet the blood pressure target.1
Target
The target blood pressure for people with CKD is consistently < 130/80 mmHg.1
This target is supported by a 2019 meta-analysis comparing groups of people with CKD: an intensive target group (average systolic blood pressure [SBP] 125) and a standard target group (average SBP 137) . It found a 21% reduction in all-cause mortality and an 18% reduction in composite CV events (myocardial infarction, stroke, CV death, heart failure, non-myocardial infarction coronary event, hospitalisation) for the intensive target group.16
Professor Ivor Katz
“The blood pressure target is important, but it can be difficult to reach. I’m okay if I don’t reach it, after trying to add on the maximum antihypertensive medications possible. The main thing is to get some decrease. Even small declines in mean arterial pressure will improve outcomes. That will still have a great impact on reducing the risk of CKD progression and cardiovascular risk.”
Guidance
The KDIGO 2021 Clinical practice guideline for the management of blood pressure in chronic kidney disease and Kidney Health Australia 2020 CKD Management in Primary Care 4th edition handbook recommend:1,6
- starting with an ACE inhibitor or ARB up-titrated to maximum tolerated dose
- adding a calcium channel blocker and/or thiazide diuretic
- adding a beta blocker, mineralocorticoid receptor antagonist (MRA) or other blood pressure-lowering medicines as appropriate for compelling indications.
Thiazide diuretic should be temporarily stopped during periods of illness or when dehydrated. It can be recommenced when the acute episode has resolved.1,11
Professor Ivor Katz
“It’s takes at least four weeks until any reduction in blood pressure is found.
“It can be difficult to know how many antihypertensives to add. But people with CKD will need more antihypertensive medications as their renal function declines. I try get as close to the target as possible without having too many side effects. I like to get the patient supporting and understanding the plan and I want to try to avoid side effects so they take their tablets.
“Once the optimal dose is found for each drug separately, then I will move onto a combination drug to reduce the pill burden. I explain this to them up front so they are not too overwhelmed with so many medications.”
SGLT2 inhibitor (dapagliflozin) – add-on therapy to slow CKD progression and reduce CV risk

Ken qualifies for dapagliflozin for CKD based on the PBS listing criteria, which include that the patient:4
- is stabilised at maximum tolerated dose for 4 weeks on an ACE inhibitor or ARB
- has an eGFR 25–75 mL/min/1.73 m2
- has a urine ACR 22.6–565 mg/mmol.
Guidance
The recommended dose of dapagliflozin for people with CKD is 10 mg taken once daily, with or without food. No dose adjustments are required for reduced kidney function, mild–moderate hepatic impairment or based on age.17,18
See the NPS MedicineWise RADAR article Dapagliflozin (Forxiga) for heart failure with reduced ejection fraction (LVEF ≤ 40%) ‘Safety’ section for detailed information about contraindications, precautions and adverse effects.
Lipid-lowering therapy
While all the above medicines have been up-titrated or added on, Ken has continued taking atorvastatin 40 mg daily for his dyslipidaemia. Ken’s dose of atorvastatin was increased from 20 mg to 40 mg daily 2 years ago.
The next step for Ken, as part of the orange clinical action plan, is to calculate his absolute CV risk score (see Australian absolute cardiovascular disease risk calculator). If Ken had moderate or severe CKD (eGFR < 45 mL/min/1.73 m2 or persistent ACR > 25 mg/mmol (or > 35 mg/mmol for females) he would already be considered high risk of a CV event (> 15% probability in 5 years) and not require this assessment.1

Ken’s absolute CV risk score is 16%, based on total cholesterol 5.6 mmol/L and high-density lipoprotein 0.9 mmol/L.
The CARI Guidelines 2022 Management of cholesterol-lowering therapy in people with chronic kidney disease recommend a statin with or without ezetimibe for:3
- adults with CKD (eGFR ≥ 15 mL/min/1.73 m2) and absolute CV risk ≥ 10%
- Aboriginal and Torres Strait Islander peoples and Māori with CKD (reduced GFR and/or albuminuria/proteinuria) and absolute CV risk ≥ 5%.
Ken meets this guideline recommendation for lipid-lowering therapy and also the PBS criteria to add ezetimibe 10 mg daily to atorvastatin, based on his eGFR being 56 mL/min/1.73 m2 and having an absolute CV risk score of 16%.19,20 He can take the two medicines as a combination product21 to reduce pill burden.
Target
No target serum cholesterol level is recommended for people with CKD1 due to a lack of evidence of benefit from titration of statin or ezetimibe to a specific low-density lipoprotein (LDL)-cholesterol target.3,22
Dr Caitlin Sum
“It’s not recommended to aim for a particular lipid target solely because the patient has CKD. A target is only recommended for other reasons such as secondary prevention of CVD.”
Guidance
The CARI Guidelines 2022 Management of cholesterol-lowering therapy in people with chronic kidney disease recommend that people with CKD should be:3,22
- provided with information about the effects of statin therapy, with or without ezetimibe, for preventing CV events and death
- started on a low-dose statin because higher doses may result in an increased risk of adverse events.
Professor Ivor Katz
“You can find that you are chasing your tail with lipid-lowering drugs aiming for a target, where you are likely to not have more benefit with higher dose but rather just more side effects.
“Many current CKD guidelines including KDIGO don’t recommend regular lipid testing but simply starting and keeping patients on highest tolerable dose. Based on the SHARP study [see above] I’m happy with simvastatin 20 mg and ezetimibe 10 mg daily. The exception may be if the patient has severe coronary heart disease and a lot of coronary events, which may need to be treated with a higher-dose statin.”
Glucose-lowering therapy

Ken doesn’t have type 2 diabetes. But if he did, the KDIGO 2020 clinical practical guidelines on management of diabetes in CKD recommend the following medicines:7
- Start as first-line therapy both metformin and an SGLT2 inhibitor, preferably one with documented kidney or CV benefits.
- If the therapeutic target is not met, add another blood glucose-lowering medicine such as a long-acting glucagon-like peptide-1 (GLP-1) receptor agonist that has documented CV benefits.
Target
The target for people with CKD and type 2 diabetes is HbA1c ≤ 7% or ≤ 53 mmol/mol.1
Guidance
Sulfonylureas are not recommended first-line treatments for people with CKD because they increase endogenous insulin production, which can lead to an increased half-life of metabolites and prolonged hypoglycaemia.8
People with CKD already treated with sulfonylureas may need to add an SGLT2 inhibitor if additional glucose lowering is recommended, and to receive benefits of slow progression of CKD and reduced risk of CVD independent of glucose-lowering effect. If an SGLT2 inhibitor is added, the sulfonylurea dose may need to be stopped or reduced to avoid the risk of hypoglycaemia.7
Some people with CKD and type 2 diabetes and an eGFR ≥ 30 mL/min/1.73 m2 may not achieve the glycaemic target with a combination of non-pharmacological management, metformin, and an SGLT2 inhibitor. Others may not be able to take these medicines due to intolerances or other restrictions, such as for people with eGFR < 30 mL/min/1.73 m2.7
Other medicines are likely to be needed in these situations. GLP-1 receptor agonists are generally preferred because of their demonstrated CV benefits, particularly among patients with established atherosclerotic CVD, and possible kidney benefits.7
Kidney impairment may require dosing adjustment or selection of an alternative medicine if a specific one is not recommended.25 See Table 2 below for guidance on selected glucose-lowering medicines.
Metformin, SGLT2 inhibitors and sulfonylureas should be temporarily stopped during periods of illness or when dehydrated. They should be recommenced when the acute episode has resolved.1,11
Table 2: Guidance for selected glucose-lowering medicines for people with CKD and reduced kidney function26,27
| Class/medicines | Reduced kidney function guidance (GFR units = mL/minute) | |
| metformin28 ♦ | Reduce maximum dose for people with stable kidney function:
| |
| SGLT2 inhibitors ● | dapagliflozin17,18 |
|
| empagliflozin29 |
| |
| ertugliflozin30 |
| |
| GLP-1 receptor agonists | dulaglutide31 ♦ |
|
| semaglutide32 ● |
| |
| liraglutide33 ♦ |
| |
| exenatide34 ♦ |
| |
| Dipeptidyl peptidase‑4 inhibitors | alogliptin35 ♦ |
|
| linagliptin36 |
| |
| saxagliptin37 ● |
| |
| vildagliptin38 ♦ |
| |
| Sulfonylureas39 ♦ | glibenclamide gliclazide glimepiride |
|
♦ GFR calculated using creatinine clearance (CrCl) in the approved product information/Australian Medicines Handbook
● GFR calculated using eGFR in the approved product information/Australian Medicines Handbook
GFR units = mL/minute; CKD = chronic kidney disease; GLP-1 = glucagon-like peptide-1; SGLT2 = sodium-glucose co-transporter 2
Conclusion
All people with CKD should first be prescribed an ACE inhibitor or ARB that is up-titrated to the maximum tolerated dose. Other medicines should be added as needed, including blood pressure-lowering, lipid-lowering and blood-glucose lowering medicines, and SGLT2 inhibitors (dapagliflozin), to slow CKD progression and reduce CV risk.
Australian and international recommendations and expert guidance for the medicines described in this article can help to:
- decide on the order of medicines to be prescribed
- understand therapeutic targets
- know what to monitor
- understand how to up-titrate dosages.
Useful resources
For health professionals
- Kidney Health Australia 2020 CKD Management in Primary Care handbook 4th edition
- KDIGO 2021 Clinical practice guideline for the management of blood pressure in chronic kidney disease
- KDIGO 2020 Clinical practice guideline for diabetes management in chronic kidney disease
- CARI 2022 Guidelines Management of cholesterol-lowering therapy in people with chronic kidney disease
- RACGP National guide to a preventive health assessment for Aboriginal and Torres Strait Islander people. Chapter 13: Chronic kidney disease prevention and management
- NPS MedicineWise Chronic kidney disease: clinical resources and tools for more guidelines, medicine management strategies and resources
- Veterans’MATES 2019 Topic 56: Medicines and your kidneys
For your patients
- NPS MedicineWise: Fact sheet How healthy are my kidneys and how do I stay well?
- NPS MedicineWise: Patient action plan My Kidney Health Plan
- NPS MedicineWise Card stack: How do I know if my kidneys are healthy and why is it important?
- Kidney Health Australia has developed patient resources Books, publications and fact
- sheets to help manage your patients with kidney disease.
- My Kidneys My Health (handbook) Available as a free hardcopy handbook and as a free mobile smart phone app
- How to look after your kidneys (fact sheet)
- All about chronic kidney disease (fact sheet).
- Make the Link: Chronic Kidney Disease, Diabetes and Cardiovascular Disease (fact sheet).
- My Kidney Care Plan helps keep track of tests and treatments. Download PDF
- This video from Kidney Health Australia explains what your kidneys do and how to keep your kidneys healthy
- Resources for Aboriginal and Torres Strait Islander peoples

References
- Kidney Health Australia. Chronic kidney disease (CKD) management in primary care. 4th. Melbourne: Kidney Health Australia, 2020 (accessed 5 January 2022).
- Johnson DW, Atai E, Chan M, et al. KHA-CARI guideline: Early chronic kidney disease: detection, prevention and management. Nephrology (Carlton) 2013;18:340-50.
- CARI Living Guideline Lipid Work Group. CARI Guidelines: Management of cholesterol-lowering therapy in people with chronic kidney disease. Westmead NSW: CARI Guidelines, 2022 (accessed 14 July 2022).
- Pharmaceutical Benefits Scheme. PBS Schedule: Summary of Changes (September 2022). Canberra: Australian Government Department of Health, 2022 (accessed 1 September 2022).
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436-46.
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2021;99:S1-S87.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2021;99:S1-S87.
- Dwyer KD, Robson B, Sum C. How to treat chronic kidney disease. AusDoc. How to Treat. Australian Doctor, 2021 (accessed 24 May 2022).
- Kidney Disease Improving Global Outcomes (KDIGO). Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19-62.
- Cheung AK, Chang TI, Cushman WC, et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int 2021;99:559-69.
- Usherwood T, Lee V. Advances in chronic kidney disease pathophysiology and management. Aust J Gen Pract 2021;50:188-92.
-
Johnson DW. Medicines shown to slow progression of CKD. Personal Communication. 4 August 2022.
- Australian Institute of Health and Welfare. Cardiovascular disease, diabetes and chronic kidney disease - Australian facts: Prevalence and incidence. Cardiovascular, diabetes and chronic kidney disease series no. 2. Canberra: AIHW, 2014 (accessed 15 May 2022).
- Xie X, Liu Y, Perkovic V, et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am J Kidney Dis 2016;67:728-41.
- Heerspink HJ, Kröpelin TF, Hoekman J, et al. Drug-induced reduction in albuminuria is associated with subsequent renoprotection: A meta-analysis. J Am Soc Nephrol 2015;26:2055-64.
- Aggarwal R, Petrie B, Bala W, et al. Mortality outcomes with intensive blood pressure targets in chronic kidney disease patients. Hypertension 2019;73:1275-82.
- AstraZeneca Pty Ltd. Dapagliflozin (Forxiga) product information. Macquarie Park, NSW: AstraZeneca Pty Ltd, 2021 (accessed 29 June 2022).
- Australian Medicines Handbook. Dapagliflozin. Adelaide: AMH Pty Ltd, 2022 (accessed 16 August 2022).
- Pharmaceutical Benefits Scheme. PBS General Schedule (September 2022). Canberra: Australian Government Department of Health, 2022 (accessed 6 September 2022).
- Pharmaceutical Benefits Scheme. General Pharmaceutical Schedule; Ezetimibe item number 8757X. Canberra: Australian Government Department of Health, 2022 (accessed 16 August 2022).
- Pharmaceutical Benefits Scheme. General Pharmaceutical Schedule; Ezetimibe and Atorvastatin 40 mg item number 10377E. Canberra: Australian Government Department of Health, 2022 (accessed 22 August 2022).
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: Evidence report and systematic review for the US Preventive Services Task Force. Jama 2016;316:2008-24.
- Palmer SC, Navaneethan SD, Craig JC, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev 2014:CD007784.
- Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181-92.
- Australian Medicines Handbook. Prescribing in renal impairment. Adelaide: AMH Pty Ltd, 2022 (accessed 16 August 2022).
-
Primary Education Advisory Committee of Kidney Health Australia. Personal Communication. 7 September 2022.
- Expert group for diabetes. Type 2 diabetes in adults. West Melbourne, Victoria: Therapeutic Guidelines Ltd., 2019 (accessed 19 September 2022).
- Australian Medicines Handbook. Metformin. Adelaide: AMH Pty Ltd, 2022 (accessed 28 July 2022).
- Boehringer Ingelheim Pty Ltd. Empagliflozin (Jardiance) product information. North Ryde, NSW: Boehringer Ingelheim Pty Ltd, 2021 (accessed 16 June 2022).
- Merck Sharp & Dohme (Australia) Pty Ltd. Ertugliflozin (Steglatro) product information. Macquarie Park, NSW: Merck Sharp & Dohme (Australia) Pty Ltd, 2022 (accessed 16 June 2022).
- Eli Lilly Australia Pty Ltd. Dulaglutide (Trulicity) product information. West Ryde, NSW: Eli Lilly Australia Pty Ltd, 2020 (accessed 16 August 2022).
- Novo Nordisk Pharmaceuticals Pty Ltd. Ozempi (semaglutide) product information. North Sydney, NSW: Novo Nordisk Pharmaceuticals Pty Ltd, 2022 (accessed 16 August 2022).
- Novo Nordisk Pharmaceuticals Pty Ltd. Liraglutide (Saxenda) product information. North Sydney, NSW: Novo Nordisk Pharmaceuticals Pty Ltd, 2021 (accessed 16 August 2022)..
- AstraZeneca Pty Ltd. Exenatide (Byetta) product information. Macquarie Park, NSW: AstraZeneca Pty Ltd, 2021 (accessed 6 September 2022)..
- Australian Medicines Handbook. Alogliptin. Adelaide: AMH Pty Ltd, 2022 (accessed 6 September 2022).
- Australian Medicines Handbook. Linagliptin. Adelaide: AMH Pty Ltd, 2022 (accessed 6 September 2022).
- Australian Medicines Handbook. Saxagliptin. Adelaide: AMH Pty Ltd, 2022 (accessed 6 September 2022).
- Australian Medicines Handbook. Vildagliptin. Adelaide: AMH Pty Ltd, 2022 (accessed 6 September 2022).
- Australian Medicines Handbook. Sulphonylureas. Adelaide: AMH Pty Ltd, 2022 (accessed 16 August 2022).
- Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019;7:845-54.
- Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776-85.
- Stefani M, Singer RF, Roberts DM. How to adjust drug doses in chronic kidney disease. Aust Prescr 2019;42:163-7.
- The Renal Drug Database. About RDD. Abingdon, UK: Taylor & Francis Group, 2022 (accessed 9 September 2022).
- Expert group for antibiotic. Renal impairment and antimicrobial dosing. West Melbourne, Victoria: Therapeutic Guidelines Ltd. 2019 (accessed 19 September 2022).